
stylish Toyota FCV-R hydrogen fuel cell 4 door sedan
supposedly coming in 2014-15 though specs have yet
to be revealed
My related essay on 'Hybrid and Electric Car Technology'
is here
Go to homepage
Hydrogen car concept
Hydrogen car problems
Hydrogen prototype cars
GM
HydroGen4 crossover
Mercedes
Benz F-Cell (class B)
Hyundai
ix35 (Tucson) specs
Hydrogen cars
in production
Honda
Clarity (6/25/17)
Toyota
Mirai (6/25/17)
Fuel cell technology
Role of platinum
catalysts
What is
the heat value of hydrogen?
Cell V vs I
My PEM membrane model
How are cells stacked?
High pressure
hydrogen tanks
Appendixes
PEM
hyrdrogen generator
Overview
of fuel cell efficiency
Thermodynamics
--- 'burning' 1 kg of hydrogen in fuel cell
CO2
from hydrogen reforming
Home
fuel cells
Reference
1 kg of H2 <=> 40 kwh or 142 Mj (HHV or 'high heat value', total
energy of combustion with O2)
(33.8 kwh or 120 Mj is 'low heat value', excludes water heat of vaporization)
(60 miles of range (typ) for current generation of hydrogen cars)
1 gal (pure) gasoline <=> 33.4 kwh or 120 Mj ('low heat value')
(1 gal gasoline <=> 2.76 kg or 6.1 lb)
1 eV <=> 96.5 kJ/mol <=> 23.06 kcal/mol
(1 kcal/mol <=> 4.184 kJ/mol)
Overview of hydrogen fuel cell cars (my emails to engineering friends)
There's another type of electric car, which is missing from my hybrid/electric car essay, the hydrogen fuel cell car. Until recently five major car manuf (now three) were promising a 'production' hydrogen car for 2014-5. I heard Leno say on a Nova show that he had been driving a GM hydrogen fuel cell car for two years and that perked my interest. So these cars have been built and they really work. Fuel cells can power a good size car, fueling time is 3 min, enough hydrogen can be stored for 250+ mile range. It's a real car, or is it? I set out to understand hydrogen fuel cell cars, and write it up.
Turns out everyone is building basically the same hydrogen fuel cell electric car: 80 kw fuel cell, hybrid size (1-2 kwh) battery in parallel, 1-2 tanks of highly compressed hydrogen in carbon fiber tanks (250 to 500 mile range). Hydrogen safety and fueling standards are mature. The car companies don't do any engineering here, they just buy a class IV tank (10,000 psi!). But a close look shows mucho problems with hydrogen cars.-- Efficiency stinks. Taking energy through a [electricity => hydrogen => electricity] cycle wastes about 2/3rd of it.-- Cost to build car far too high. The fuel cell uses platinum for catalysts on both electrodes and in decades of research no one has found a practical replacement, though the amount needed had dropped. An single cell under load only puts out 0.7V, so it takes 400+ cell in series to get 300V. The fuel cell is thus a large stack of thin cells each of which needs a flow of hydrogen in/out, oxygen in/water out, and cooling antifreeze, because this sucker is going to put out about 33 kw of heat. An 80 kw stack is, surprisingly, not that big (suitcase size) or heavy, but no one knows (yet) how to build it cheaply. The carbon fiber hydrogen tanks also cost thousands.
-- Hydrogen fueling/transport nightmare. Unlike with electric cars where you can home recharge and outside fueling stations can come later. Home hydrogen refueling is impractical, there has got to be outside hydrogen 'gas' pumps. This can work for central fleet cars or buses, but for general use it's a classic chicken and egg problem. Even worse there appears to be no economical way to transport large quantities of hydrogen to a dispersed network of hydrogen stations. Compressed truck capacity is low, liquid hydrogen transport/storage means another 10-20% efficiency loss (liquid hydrogen is 20C above absolute zero), hydrogen embrittles metal, so standard tanks and pipes have problems with hydrogen. Hydrogen stations may need to make their own hydrogen locally via electrolysis, and this step alone wastes 25-35% of the electrical energy.
Questions: Does hydrogen technology need time to develop? What would be the cost of a fill up?IntroThe bad news is the technology is pretty well developed. Bush in 2002 announced a billion+ spending plan on hydrogen technology, so the government has been spending big bucks on hydrogen cars for over a decade. The membrane that is at the heart of the fuel cell was developed in the 60's for the space program, and fifty years later it is EXACTLY the same, same catalyst too.
What is new over the last decade is technology for compressed hydrogen tanks. Newer cars have switched from heavy metal 5,000 psi tanks to much lighter carbon fiber 10,000 psi tanks, but there is no mention of any higher pressure tanks under development, so 10,000 psi appears to be the limit, and besides it takes years for safety standards and tests to be developed to insure these cars aren't rolling bombs. So the new high pressure carbon wrapped tanks appear to be safe and light, but the problem is carbon fiber technology is now pretty mature (think new planes), but expensive, speculation is even in production thousands of dollars per tank. The high cost per tank is why I suspect some of the prototype hydrogen cars have thrown away the inherent advantage they have over electric cars, putting in only one 250 mile tank instead of two for 500 miles. Two tanks are a squeeze, but can be fit in below the floor.
The irony on efficiency is that every hydrogen car will advertise how efficient they are by only focusing on the car (60% fuel cell vs 20% combustion engine), but a table in my essay shows that for the same amount of grid electricity a battery electric car will travel x3 further than a hydrogen electric. They also will claim no pollution ('Only H2O comes out'), but if the hydrogen is generated by reforming natural gas, as it mostly is, then CO2 emission is same (or higher) than a regular car.
The cost numbers are very hard to pin down until a production car appears. But the speculation is (supported by data from government labs) that the prototype hydrogen cars on the road now might have cost as much as one million dollars apiece to build, and that car makers are struggling to bring the cost down to 100k. The lowest target ever mentioned is 50k.
It doesn't really matter what the cost of hydrogen is if you can't find a pump, but hydrogen, now only made in small quantities, is far from cheap. The fact that advocates never mention what the cost of a tank of hydrogen will be is telling. When skeptics looks at the manufacturing and distribution costs of hydrogen, they figure a tank of hydrogen (without subsidies) would be far more than a tank of gasoline.
(Nov 2013 update)It's going to be a long time before any of us will be shopping for a hydrogen car.
The NYT car editor recently described his experience driving a hydrogen car. He has been driving a hydrogen car (prototype Toyota) for months refueling at the only hydrogen pump in northern CA, which is 6 miles (15 min) from his home. His car has four 10,000 psi hydrogen tanks (Toyota will reduce that to two in the production car). He typically 'tops off his tanks' with 4-5 kg of H2 (eq to 240-300 mile range) paying $12-13/kg for a refueling cost of $48-$65. (No mention of subsidy, but you can bet your first born that the 1-3 million dollar hydrogen gas station was built mostly, or fully, with government money.) The non-hydrogen version of the car he is driving he notes gets 27 mpg, so he figures he is paying the rough equivalent of $6/gal.
Hydrogen fuel cell car
A hydrogen
fuel cell car is an electric car, meaning the wheels are powered only by
an electric motor. But instead of a big battery (like the pure electric
Tesla), it has a hydrogen powered electric generator, sort of a flow through
battery, called a fuel cell, that runs on a flow of hydrogen from an onboard,
high pressure hydrogen tank. However, hydrogen is not a 'fuel' in the traditional
sense. It is not dug up or grown, it's 'manufactured' (from water!) with
all its energy content coming from other fuels or energy sources. This
is crucial point in assessing the efficiency claims of a hydrogen car.
To provide regenerative braking and assist in acceleration these cars also
have a small (hybrid size) battery connected across the fuel cell.
In principle a hydrogen fuel cell car solves the classic two problems of the electric car: range and fueling time, but it brings its own (serious) problems. It is expensive to make, puting energy into hydrogen and then taking it out is not efficient, and to be practical nearby hydrogen pumps are required, because home charging with hydrogen is not an option.
The existence of a few hundred hydrogen powered cars on the road for a few years means:
1) Reasonably reliable fuel cells suitable for a car have been developed
2) A way to store enough hydrogen in a car for reasonable range has been
developed
3) A way to refill the tank quickly with hydrogen has been developed
I remember reading an article in Scientific American about hydrogen cars a few years ago listing all the problems and in tone was quite negative. In 2013 the Volkswagen CEO is supposed to have said, "fuel cell vehicles have no future", which is pretty strong. Ford has an essay on their site listing all the reasons why a production hydrogen car doesn't make sense now, and why they are only supporting R&D. Obama's energy secretary and Steven Chu, a Noble prize winning physicist, has had negative things to say about the hydrogen car and lowered hydrogen funding when he came into office.
Yet quite a few car manufacturers are sanguine (in public anyway) about the future of hydrogen for vehicles and continue to support the development of a hydrogen powered fuel cell car. The big players are GM (US), Honda, Toyota (Japan), Mercedes Benz (Germany) and Hyundai (Korea), and until recently all of them said they were planning to introduce a production hydrogen car to the market in 2014-15. The US government has been spending a lot of money in support of hydrogen vehicles since the early Bush days with a lot of R&D work by National Energy lab (NREL). Wikipedia 'hydrogen cars' is kind of rambling, but it hints at the problems.
The question remains open at this point (2013) as to whether or not hydrogen cars are ready to soon transition from R&D prototypes to sale in the market as did hybrids and electrics before them. A bad sign is that GM and Honda have just bailed, now they are saying in 2020 (maybe) they will introduce a production hydrogen car. This leaves the remaining players for a hydrogen fuel cell car in 2014-15 as Mercedes, Toyota and Hyundai. Hyundai seems to be leading the pack saying production has already begun and promising to build 1,000 cars by 2015. Toyota is planning to introduce their production hydrogen model in late 2013 (at an auto show).
When a production car? (9/13)On the positive side lots of technical progress in hydrogen cars has been made in recent years and several manufacturers have already done small 'production' runs, so there are now 500 to 1,000 hydrogen cars on the road. A lot of engineering has been done by car companies on fuel cell cars, and testing houses have developed fuel tanks standards and refueling standards. Wikipedia says 20 or so different prototype models have been engineered in recent years, but not a single fuel cell car is for sale now from any manufacturer, though a limited number of hydrogen cars are being leased in a few cities where there are hydrogen refueling stations, which in US means mostly LA. So what are the problems, I set out to find out.
The date for a production hydrogen car is a moving target, as the date approaches like clockwork so do news stories that a few more years of development (read cost reduction) are needed. The press announcements follow a pattern: xx and yy car companies announce they are teaming up to work jointly on hydrogen technology (of course this reduces their R&D costs for both of them), and at the same time they jointly predict a production car with the date moved out a few years beyond 2015. In 2009 all five of the major players were saying a production hydrogen car by 2015.A few months ago Honda and GM teamed up, and their target date for a car moved to 2020. Now I find a recent news story that the serious hydrogen player Daimler/Mercedes has teamed up too. Daimler is joining with Ford and Nissan-Renault to develop a hydrogen car, and (guess what!) a new target date for production car coming out of this work is "as early as" 2017. While I still read Toyota may come to market with a hydrogen car in 2015, no one has a clue what it will cost with guesstimate prices ranging from 50k to 135k. (In an important sense it probably doesn't matter what the first models cost, since with only a handful of hydrogen refueling stations only a few hundred (at best) will ever be sold, if they are even sold at all and not leased.) Now Toyota has announced they too are teaming (with BMW) and this team has a target date for a production hydrogen car of 2020.

stylish Toyota FCV-R hydrogen fuel cell 4 door sedan
supposedly coming in 2014-15 though specs have yet
to be revealed
Hydrogen car concept
Conceptually
the modern hydrogen powered fuel cell car is quite simple, another electric
car. You might think a hydrogen car is just an electric car where a fuel
cell and a tank of hydrogen replaces the batteries. Car company marketing
depts are fond of making this claim, but it's not quite true. A hybrid
size (1-2 kwh) lithium ion battery is also needed to boost acceleration
and provide regen braking, so all the hydrogen fuel cells cars are also
quasi-hybrids. The fuel cell itself has zero moving parts. You run
hydrogen gas, air and liquid coolant (anti-freeze) through this fairly
small, fairly light (150 lbs), honeycombed box and out comes up to 80-100
kw @ 300 VDC (plus a lot of waste heat). It's like a nominally fixed voltage
battery whose current (0 to 333A DC) can be modulated by controlling the
flow of hydrogen, which is the job of a valve mechanism linked to the gas
pedal.
Throw a hybrid size (1-2 kwh) lithium ion battery across the fuel cell and no worries about the fuel cell's response time, extra power to aid acceleration, a place to put energy recovered from regenerative braking (fuel cells don't run backwards), and when the car is stopped or at very low speed the fuel cell can be shut down and the car and its accessories run from the battery. Like all cars some pumps and aux systems are needed. An air compressor is required to increase the oxygen density, a 'water' (coolant) pump and radiator are needed to cool the fuel cell, and a moisture control system is needed to keep the membrane hydrated ('wet'). No pump (I think) is needed in the hydrogen path, since the hydrogen is under high pressure in a tank. The electric motor and its single inverter (no transmission) are the same as any electrical car. The NYT car editor, who regularly drives both a pure electric car (Leaf) and a hydrogen/electric car (prototype Toyota), notes unlike the near silent operation of his electric car in his hydrgen car he can hear the "hiss of an air compressor".
Hydrogen car has hybrid like features, but it is not a hybridFuel cell
It's tempting to call the hydrogen car with its fuel cell and hybrid size battery a 'hybrid', Toyota does and I do sometimes, but it's power path is really very different from a classic hybrid. In a classic hybrid, like the Toyota Prius, there are two sources of mechanical power, a combustion engine and electric motor with a complex gear system and controls to vary the power split between them for powering the wheels. A hydrogen fuel cell car has none of this.In a hydrogen fuel cell car the wheels are driven (at all times) by an electric motor as in any electric car. A fuel cell is nominally a fixed source of voltage (about 300V), but it only put out power, it can't absorb it. A relatively small (1-2 kwh) battery is put across the fuel cell to in essence make it a better voltage source, to help supply current for acceleration and absorb current when the vehicle is braked. Thus a hydrogen fuel cell car has two sources of electrical current (in parallel) to power its single electric motor, which drives the wheels.
The fuel cell is essentially a flow through battery. To get 300V requires around 440 cells in series, since each cell only develops about 0.7V. Each cell has to be provided with a flow of hydrogen gas and (compressed) air that has to diffuse into the anode and cathode of each cell while not blocking the electron flows from cell to cell. The gases come in via channels cut into what is called a bypass plate (hydrogen on one side and air on the other) that is also a electrical terminal. It must be strong, thin, have very low resistance (since it carries the full 300A) and be impervious to gas diffusion. Nobody yet knows how to manufacture this thing economically in volume and each fuel cell has 440 of them.
The anode and cathode are the outer two layers of a triple sandwich with a PEM (proton exchange membrane) between. The PEM serves as the electrolyte of the battery, physically it is solid membrane that is hydrated, i.e. it includes water. It is designed to only pass only protons, which is what remains of the hydrogen gas after a reaction at the anode, catalyzed by platinum, splits H2 and strips off its electrons. The reaction at the cathode, where the O2, electrons and protons meet to form H2O, is also catalyzed by platinum. The amount of platinum in the fuel cell is one of the major drivers of its cost.
The PEM membrane is the heart of each cell, it converts more than half of the energy from electro-chemical 'burning' of hydrogen to electricity. However, electro-chemistry dictates that a substantial fraction of the energy released by the hydrogen 'burning' is released as heat, and in the whole fuel cell that's a lot of heat (tens of kw), so hundreds of liquid coolant channels are needed thoughout the fuel cell to cool each cell. Thus a fuel cell is a stackup of complex cells (each with two gas flow paths, a liquid coolant path, an electrical path and a thin film membrane sandwich with platinum) made with materials that are either expensive or hard to manufacture. No wonder it is costly. The modern car fuel cell is relatively small and light, and some of the prototype cars put it under the floor of the passenger compartment. Really, its cooling system works that well?
Battery
The battery across
the fuel cell works like the battery in a hybrid car and is the same size
(1-2 kwh). It outputs high peak current to aid acceleration reducing the
peak current in the fuel cell, it can run the car at low speeds with the
fuel cell shut down, and it is required if the car is to have regenerative
brake, because fuel cells don't run backwards, you can't put in current
and get out hydrogen!
Tank
About a decade ago
very high pressure (10,000 psi) light, carbon fiber hydrogen storage tanks
were developed, and in the last ten years they have become standardized,
all build to safety standards developed by outside bodies. The auto companies
don't need to engineer the hydrogen tanks, they just specify a class IV
tank of the size they want.
Hydrogen car problems
When you dig
below the marketing hype you find the hydrgen car has three or four big
problems:
1) Manufacturing cost --- fuels cells and hydrogen storage tanks are expensive
2) Efficiency --- huge losses in fuel cycle [electricity => hydrogen =>
electricity]
3) Chicken and egg problem --- need hydrogen refueling stations, no recharging
at home
4) Range --- range is moderately good (240 miles), but a 2nd tank (used
in a few prototypes) that would boost range
to far longer than battery powered electrics is for some reason (maybe
cost) often left out
1) Car manufacturing cost --- A 64 dollar question is how cheaply in the future can the fuel cell and the hydrogen tanks be built. There are strong hints that the manuf cost of current hydrogen cars are prohibitively high, maybe 100k+ each, dwarfing the battery cost problem in electric cars. The problem is the high manufacturing cost of the fuel cell and (perhaps) the high pressure carbon fiber tank(s) used to store the hydrogen.
On the fuel cell, it is unclear to what extent its high cost is driven by the complex honeycomb structure of the fuel cells (with its 400 or so cells each having large area thin diffusion sandwich with access to two gas flows and cooling) or by the materials used in the cells, specifically the amount of platinum, which is the key catalyst needed to make the reactions go (or go fast enough for a 100 kw fuel cell). People have been looking for cheaper, robust catalysts forever, but all the fuel cell cars use platinum (platinum has deep roots in chemical cells). Platinum appears to be the only catalyst that is now practical.
20 gm of platinumOn the tanks, these are state of the art carbon fiber tanks operating at very high pressure (10,000 psi!). It is very difficult to pin down their cost, but there are hints that these tanks may now also be very expensive, possibly between 5-10 thousand dollars each. I also read that carbon fiber technology is a mature technology, so R&D work is on trying to reduce the amount of carbon fiber needed. Detail tank safety standards exist, and they specify that 'burst' (read bomb) pressure be about 230% of the max operating pressure.
I found a Scientific American article from a couple of years ago that said a typical car fuel cell uses 30 grams of platinum, worth about 4k. A 2013 NREL report says the amount of platinum in fuel cells is now down to 20 grams. Ok, not cheap, but not a show stopper in a world of 100k cars. But the numbers don't add up. I checked the price of platinum and the raw cost for 20 grams of platinum metal is about 1.1k (plus I am sure some processing costs to expand its surface area). A decade's work has already reduced the amount of platinum needed by a factor of 4. Since it is the surface of the platinum catalyst that does the work, nano-technology has the potential to further reduce the amount of platinum.An NREL report estimates that the fuel cell production costs for very high volume production (1/2 million car/yr) might be reduced to little under 5,000 dollars for a 100 kw fuel cell! (To put this in perspective electric car sales now are now in the range of ten thousand/yr.)
2) Efficiency --- Whether a hydrogen car is efficient, or inefficient, depends on what you compare it to. Overall system efficiency of using hydrogen as a fuel in a vehicle is terrible, well certainly not good. There are three large efficiency losses in the hydrogen system: 35-45% loss in the fuel cell, 25% lost in electrolysis making hydrogen, and another 10% loss compressing it, plus it costs more to transport hydrogen than electricity [.41 = .60 x .75 x .9]. Thus the electrical energy that comes out of a fuel cell is something like 30-40% of the energy that was used to 'manufacture' the hydrogen, more than half (perhaps 2/3rds) of the starting energy gets lost in the double conversion: [electricity => hydrogen => electricity].
3) Refueling stations --- Unlike electric cars there is a terrible chick and egg problem with hydrogen cars. For electric cars public charging stations are a convenience, but not an absolute necessity. You can have an electric car anywhere by charging it at home, slowly with just a ordinary outlet or more quickly with a home charging station that costs a few k to install. No such option exists for hydrogen cars. The only place (unless your rich like Leno!) to get hydrogen fuel is at a hydrogen 'gas station'. When only a few hydrogen stations exist, like now, you need to live quite close to one of these stations to drive a hydrogen car. (Even a 30 mile trip to a refueling station and back shaves 25% off the usable range of current hydrogen cars (240 => 180 miles. And someone argued why drive even 15 min to recharge with hydrogen when gas stations exist on every corner). For fleets with their own central refueling station full range is available, this is one of the reasons hydrogen is used on some buses. So for quite a while hydrogen cars are going to be restricted to large cities (where it doesn't get too cold). Mercedes now had five refueling stations in US (4 in LA and 1 in SF for 70 cars to be delivered in 2012). These initial cars are not being sold, they are being leased for 10k/yr (3 yr lease).Honda currently advertises that their new "FCX Clarity's fuel efficiency is three times that of a comparable, modern gasoline-powered automobile." Fuel cells in cars have long been advertised as 'more efficient' than combustion engines. True, but misleading. Combustion engine efficiency is terrible, in the 20% range, but with gasoline this is not really a problem, since a small tank of gasoline packs so much potential energy and is so cheap.The natural competitor to the hydrogen fuel cell car is the (pure) electric car. Most of the electrical energy put into a battery comes out with an efficiency something like 80+%, far higher than the hydrogen fuel cycle [electricity => hydrogen => electricity]. A chart below shows the electrical grid load (per mile) of a fuel cell car is about x3 higher than an electric car. So from this perspective a hydrogen powered car is quite inefficient. A huge and fundamental disadvantage.
Slate on hydrogen efficiency
In summer 2013 an article in Slate comparing hydrogen and electric cars stated that: "Hydrogen fuel-cell cars are more efficient than electric vehicles". This is hogwash. What they are referring to is that the weight of the drive train of a hydrogen car [maybe 500 lbs total for the fuel cell, hydrogen tank(s) and hybrid size battery] is about half the weight of the big battery in the Tesla model S. This does affect mileage a little (all these cars have regenerative brakes), but this weight difference is totally swamped by the roughly 40% heat loss of the fuel cell (not to mention the losses making, transporting, and compressing the hydrogen) vs the 10% or so loss in a battery as it discharges.
Make hydrogen at home?4) Range --- The range of most of the hydrogen cars likely to come to production is reasonable (240 miles from 4 kg of H2), but it's probably not high enough to compete against the best of the electric cars like the Tesla Model S, which is comparable. Hydrogen cars need an advantage over electric cars to compensate for the difficulties in obtaining hydrogen. The problem is that tank pressure has maxed out at 10,000 psi, so for more range car manufacturers have to find room below the floor (a design decision) for either a bigger tank or 2nd tank to hold more than 4 kg of hydrogen. 6-7 kg is what is needed for a 400 mile range, and this is what early development of hydrogen cars always assumed the cars would carry. This can be done, a few prototype hydrogen cars have fit in below floor a 2nd half size tank (360 mile range) or a 2nd full size tank (480 mile range), so it is feasible. Then why the marginal range? One reason may be that besides space constraints the current cost of high pressure carbon fiber tanks is really high, thousands of dollars. Also double your range and you have doubled your refueling time, so it may no longer be possible for marketing to clam that the car can be refueled in the same time as a regular car.
Turns out that small hydrogen generators that make hydrogen from water via electrolysis are sold. They would easily fit in a garage (see spec below). The smaller unit (32" high) runs at 3.5 kw on 220 VAC and will make 1.3 kg/day (enough for 80 miles of driving). A double height unit running on 440 VAC at 11.5 kw can make 5 kw/day, or enough to fill the tank in less than 24 hours. Cost?Unfortunately this is only part of what would be needed for a home hydrogen filling station. You also need a big tank to store the hydrogen and a powerful compressor (no doubt expensive) that can raise the pressure by x700 times (to 10,000 psi)! Not only that but you probably need a refriderator too, because hydrogen at pumps is pre-chilled to limit the max temperature in the car's carbon fiber tanks as the gas is compressed. There would undoubtedly be significant safety and regulatory concerns with keeping a tank of hydrogen. I doubt home hydrogen charging is remotely practical. Yup, I found this company, ITM Power, is looking into selling the complete package, and sure enough it's a mother! (2nd link below)
http://www.itm-power.com/product/hpac-10/
http://www.itm-power.com/wp-content/uploads/2012/04/70MPaHomeRefuelling-Vancouver-160511.pdf
Looking way into the future
On the other hand,
as advocates always point out, looking far ahead (50-100 yr?) when
fossil fuels are very expensive and the grid is powered (mostly) from renewable
energy sources a practical hydrogen vehicle technology could be very important.
It would provide an alternative fuel for cars and trucks (forever!) with
no CO2 emissions into the atmosphere. While hydrogen cars have half (or
less) the efficiency of battery powered electric cars, it is possible that
the battery technology will never develop, that electric cars will continue
to be hobbled by inadequate range and charge times that are too slow.
When fossil fuels are gone, it might be possible (affordable?) to use grid renewable electric energy go make gasoline (a hydrocarbon) for vehicles. If the source carbon is plant material, at least it would be (nominally) CO2 neutral and not affect the atmosphere, but still with the low efficiency of combustion engines about x3 more energy would need to be put into the manufactured gasoline than into hydrogen.Fuel cell
Hydrogen storage in vehicle
A lot of people
are working on materials that can adsorb and release hydrogen, over
the years I have seen a lot of 'breakthrough' stories. The goal is enough
hydrogen for good range to be stored in reasonable volume without high
pressure. But all the current crop of prototype hydrogen cars don't use
use any tank insert material, just really high pressure. Sure brute force,
but it's simple, it does work and is (hopefully) safe, they get
240 miles with a single tank under the floor. How much these high pressure
tanks cost is another matter, and how dangerous they are really remains
to be seen. (Are you riding in a bomb?) Refueling time is not bad,
about 5 min. Durability of the many recent prototypes build seems to be
good, just 10% degradation at 75k miles. One concern which is hard to pin
down is how pure the hydrogen needs to be. Hydrogen made by reforming methane
has trace CO and COs poison's some catalysts.
 .
.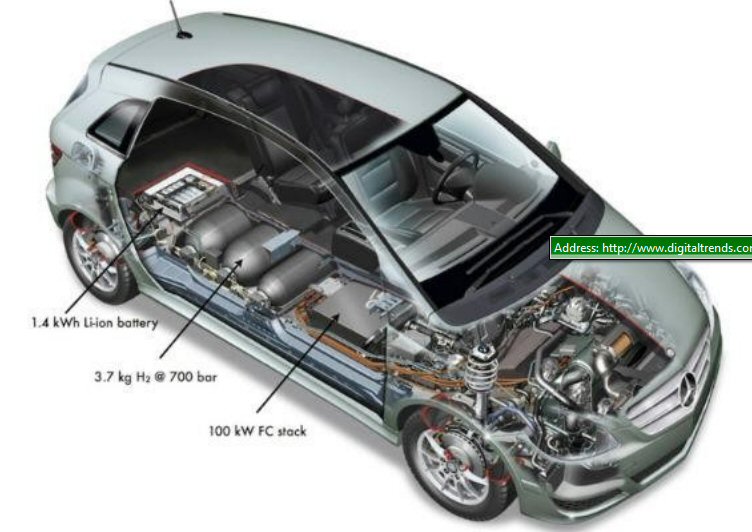
Multiple (high pressure) hydrogen tanks of the GM
HydroGen4 (left) and another model are visible here
Cost and CO2 emissions of hydrogen 'manufacture'
While Jay
Leno points out that a hydrogen car is zero emission (as is an electric
car), but pure hydrogen doesn't exist in any quantity on earth, it must
be manufactured. This requires energy (from fossil fuels) with of course
CO2 emissions. One 'expert' on Wikipedia stated the way hydrogen is usually
made, steam forming, puts out as much CO2 per mile as burning gasoline.
Here's a chart showing that the energy losses associated with 1) generating hydrogen by electrolysis (separation of water into O2 and H2 with electricity), 2) energy required for compression to high pressure, and 3) fuel cell efficiency (assumed here to be 50%) result in only 23% of grid power coming out of electric motor shaft vs 69% for an electric car, so they conclude an electric car is x3 more efficient than a hydrogen car. Numbers like this are always a little squishy, but they are in the right ballpark, and I think this is the right way to compare a hydrogen and battery powered electric car.
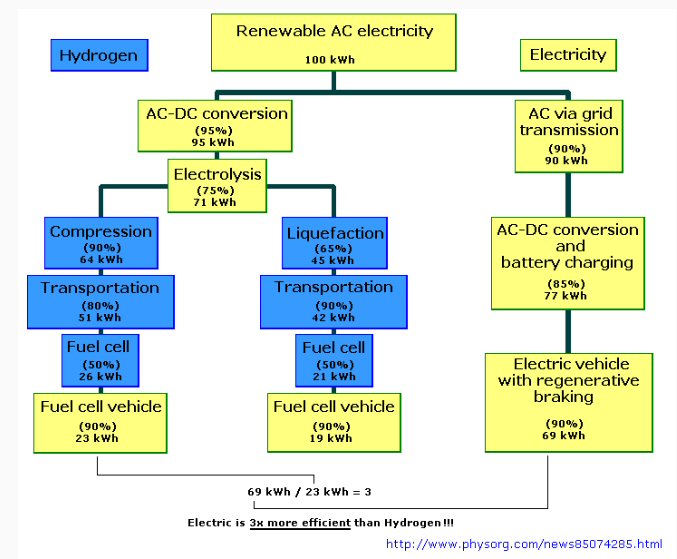
These numbers are confirmed by Wikipedia 'fuel cells'

GM/Opel hydrogen car (2008) -- HydroGen4
fuel cell structure
440 cells (440 cells x 0.7V/cell (nom) = 308 VDC)
fuel cell power
73 kw
battery (hybrid)
1.8 kwh/35 kw (NiM hydride)
electric motor
(108 kw) (cont, 3 phase asynchronous) (yes, an induction motor)
motor torque
320 nm
hydrogen tank pressure
10,000 psi (700 bar)
hydrogen capacity
4.2 kg (168 kwh eq = 40
kwh/kg x 4.2 kg)
wheelbase
113 in
range
200 miles
max speed
100 mph
acceleration
12 sec (0 to 60 mph)
50% efficiency implied
Note a full tank
of 4.2 kg of H2 in HydroGen4 provides a 200 mile range. The (combustion)
energy of 4.2 kg of H2 (in units normally used for batteries) = 168 kwh
(see below). This is just about double the energy storage of the
largest battery (85 kwh) of the Tesla model S sedan, which has a 200+ mile
range (Tesla claims 265 miles). While the Tesla is a slightly smaller car,
on the other hand its batteries cannot be fully discharged. Bottom line
the fuel cell in the HydroGen4 appears to be converting only about half
the fuel energy of the hydrogen in its tanks to electricity, consequently
the other half must be coming out as waste heat. This is generally consistent
with the Wikipedia article on 'Fuel Cell' that says fuel cells are generally
40 - 60% efficient (50-70% for PEM type), or if waste heat is captured
for use up to 85% efficient.
Mercedes
Benz F-Cell (class B)
The Mercedes
Benz hydrogen car is a four door sedan, slightly smaller than the Honda
and GM, but inside it's basically the same car. A proton exchange fuel
cell combined with a hybrid size lithium ion battery to output 100 kw.
Compressed hydrogen as the fuel. The detail Mercedes spec shows the tank
pressure as 10,000 psi (like Honda) holding 3.7 kg of hydrogen which provides
a range of 240 miles (60 miles/kg), but news stories speak of two models
one with a 5,000 psi tank and another with 10,000 psi tank with different
ranges. In 2011 Mercedes manufactured 200 of these Class B hydrogen cars
for use in Germany and in CA.
fuel cell structure
Proton Exchange Membrane
fuel cell power
70 kw (90 kw) peak
battery (hybrid)
1.4 kwh (Li ion, liquid cooled)
battery power
30 kw (10 kw) (peak)
electric motor
100 kw
motor torque
290 (320) nm
hydrogen tank pressure
10,000 psi (700 bar, three tanks)
hydrogen capacity
3.7 kg of H2 (< 3 min to fill tank)
wheelbase
109.4 in
weight
3,980 lb
range
240 miles (some Mercedes ads say 190 miles)
max speed
106 mph (170 km/hr)
acceleration (0 to 60)
11.3 sec
cold start
-13F (-25C)

Mercedes Benz F-cell, class B, hydrogen fuel cell
car
Under the floor are the fuel cell (1) and three hydrogen
tanks (2),
the lithium ion battery is under trunk (3)
Note large radiator in front.
The price looks
like a fiction. I found a dedicated Hyundai
hydrogen Tucson site. It clearly says the car cannot be purchased and
no purchase option will be available at the end of the lease.
-----------------
Summer
2013 new stories are than Hyundai plans to make 1,000 of its ix35 hydrogen
fuel car by 2015, so it's arguable that Hyundai has the first 'production'
hydrogen fuel cell car. Specs below are for 2008 Hyundai iBlue, which appears
to be close to the ix35, but may not be exactly the same. (Some news stories
call the car entering production the Hyundai Tucson, but a Hyundai video
shows the car nameplate is ix35.)
fuel cell structure
Proton Exchange Membrane
fuel cell power
100 kw
battery (hybrid)
0.95 kwh (Li ion) (60 Ah x 15.8V?? = 0.95 kwh)
battery power
24 kw
electric motor
100 kw (induction, in spec, but NYT article says PM)
motor torque
300 nm (221 lb-ft)
hydrogen tank pressure
10,000 psi (115 liters)
hydrogen capacity
(5.6 to 6) kg of H2 (eq to 340-360 mile range)
5.6 kg (12.4 lb) (Car & Driver)
fill up time
10 min
wheelbase
103.9 inches
weight
4,150
range
370 miles (yes, finally someone has put in more tank!)
265 for 5.6 kg at 10,000 psi (Tucson spec)
max speed
100 mph
acceleration (0 to 60)
12.5 sec
cold start
(-20C)
On their fuel cell technology Hyundai say this:
"The Hyundai fuel cell has one major difference compared to its competitors: The Hyundai fuel cell uses near ambient air pressure to provide oxygen to the fuel cell stack compared to fuel cell systems that use compressed air. Compressing air requires additional energy. Hyundai's design results in low parasitic loss in the oxygen supply, which leads to high fuel efficiency and reduces power consumption by 50 percent. This setup also reduces noise in the cabin."
 .
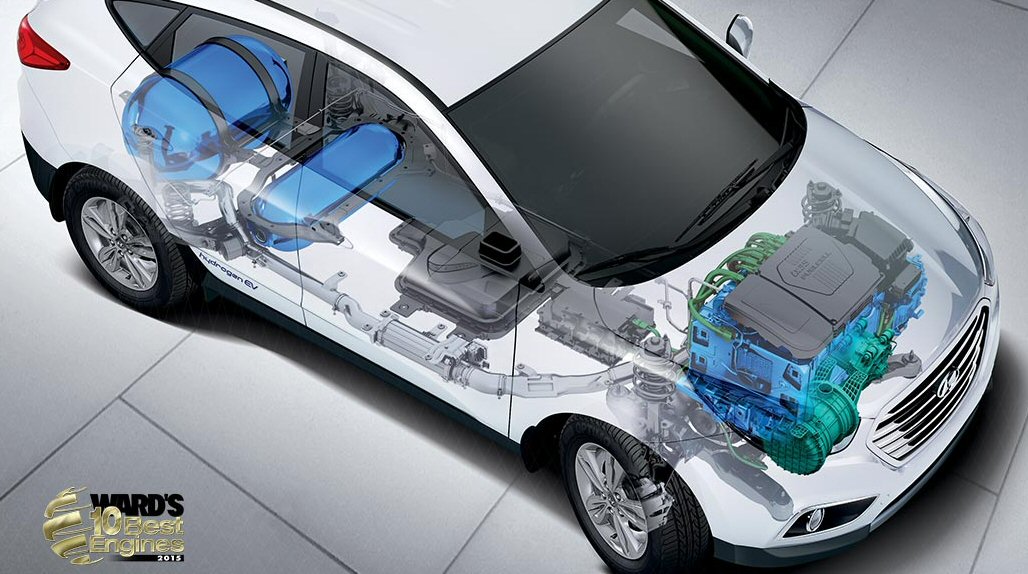
. 
 .
. 
(left)Screen capture of an ix35 Hyundai video
(right) Tucson hydrogen gas port (I find it curious
the pressure is not marked)
(source -- http://www.hyundai.co.uk/about-us/environment/hydrogen-fuel-cell)
The two Hyundai
crosssections above appear to show that the car has two hydrogen tanks:
full size (4 kg) and (probably) half size (2 kg), which is consistent with
its (projected) 360 mile range.
------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Hydrogen cars in production(update
6/25/2017)
In July 2017
issue of Scientific American I came across an ad "introducing" a hydrogen
car from Toyota that is being sold called the Toyota Mirai. A little
googling finds March 2017 article that says hydrogen cars are real. It
discusses two production (?) hydrogen cars: Hyundai 2017 Clarity and the
Toyota Mirai. Well both can be bought (at least in CA) or can they? That's
a step forward. Looking at this hydrogen car essay I find I wrote
technically about both of these cars cars several years ago.
Can they be bought? (7/25/17)
While Toyata says
they will sell you one for 57k, a mar 2017 LA Times article says
they have 1,245 cars and nearly all of them are leased. Honda is not
selling the Clarity only leasing it. There are now 27 hydrogen fueling
stattions, most in southern CA.
Honda Clarity vs Toyota Mirai
These two
early 'production' hydrogen cars are very similar, about the same size
and weight, both store hydrogen at 10,000 psi. Both claim somewhat over
300 mile range (sedate driving). The Honda uses a Li-ion hybrid battery
(unspecificied KWA), while the Toyota uses the older nickel metal hydroid.
Fuel cell power rated 103 to 114 kw, motor torque (PM motors) 300 to 330
nm. Toyota as in their hybrids boost the stack voltage from 340 VDC to
650 VDC. Honda does not mention a voltage booster. Honda has a sport mode,
apparently Toyota does not.
Toyota is a 4 passenger car, while Honda is a 5 passenger car. This may be because (as an LA Times story says) that Honda has a better layout with the stack and key power components under the hood and not between the two back seats (like the Toyota?).
Honda Clarity---
'production' hydrogen car (6/25/17)
Honda has
done pretty much what GM is done, made a few dozen hydrogen fuel cell cars
('FCX Clarity') and put them on the road, many near LA where there is refueling.
(I read it cost 1 million and two years (permitting) to build a hydrogen
refueling station!) Like the GM the Honda fuel cell car is are highly engineered,
a real car. Honda had gone through three or four generations of hydrogen
fuel cell cars over a decade making big advances in the engineering. For
example the fuel cell is now much smaller and lighter than in earlier generations.
Here's Honda's marketing claim:
"Significant advances include exhilarating performance, futuristic sedan packaging and a driving range of up to 240 miles". FCX Clarity's fuel efficiency is three times that of a comparable, modern gasoline-powered automobile."Honda Clarity specs
fuel cell structure
Proton Exchange Membrane
fuel cell power
103 kw (346 VDC)
battery (hybrid)
(Li ion 288 VDC)
electric motor
174 kw (pk, 3 PM phase synchronous)
motor torque
300 nm (221 ft-lb, (0 to 3,500 rpm)
hydrogen tank pressure
10,000 psi (70 Mpa, xx tanks)
hydrogen capacity
5.46 kg
refill time
3-5 min
wheelbase
108.3 in
weight
4,134 lb (stack 114 lb)
range
366 miles in sport mode more like 260 miles)
EPA milage eq
68 mph
max speed
100 mph?
acceleration
Honda 'Clarity' specs: http://world.honda.com/FCXClarity/specifications/index.html

Honda Clarity, hydrogen fuel cell prototype (2010)
Praise for inside of Honda Clarity by LA auto writer,
and he points out its total impractability
http://grist.org/article/time-to-combust-the-hydrogen-fuel-cell-vehicle-program/
Honda has built 200 and has 25 or so of their latest generation Clarity on the road, in USA clustered around LA where there are hydrogen refueling stations. This is the 3 or 4th generation of their fuel cell cars over a decade. The single high pressure hydrogen tank is behind the rear seats. The fuel cell (surprisingly) is located in the center divider. One reason is that is needs to stand up because they find vertical gas flow (water down flow due to gravity) works better than their earlier horizontal design.
Other design features: A noisy compressor is used to compress the air going into the fuel cell. The fuel cell has substantial liquid cooling not unlike that of a standard engine, except internally inside the fuel cell the flow divided down like capillaries into hundred or more tiny paths so each cell is cooled.
Fuel cell designCost
A detailed Honda write up of the fuel cells shows it is quite fine textured honeycomb marvel. With a 288 VDC bus and 0.7 V (nom/cell) something like 400 cells in series are need and are stacked up in series inside the fuel cell box. Two gases (H2 and compressed air) flow into the top and flow vertically through many fine channels and out the bottom. The liquid coolant flows horizontally around these gas tubes. The working guts of each cell is a thin triple sandwich [electrode - separator - electrode]. Platinum (required catalyst) is either in or on the surface of each electrode. The hydrogen and oxygen gas diffuse through the two electrodes, and the protons (hydrogen nuclei) diffuse through the PEM separator, which is designed to only pass protons. All three of these elements need to have very low resistance, because with a 100 kw @ 300V each cell element is conducting 333A.What the detail cell geometry is I haven't seen, but it is clearly one of the key feature of the fuel cell. It's must be quite tricky, the current is high in the electrodes, but each electrode must be bathed in its gas, plus (isolated from the gases and current) the liquid cooling has to pass by each cell too to pick up the waste heat. This is where a lot of the engineering has gone in over the last decade. Honda has graphs showing how the size and weight of the fuel cell had dropped by a factor of two or three as they have optimized it. The 100 kw Honda fuel cell is something like 150 lbs, which is very light. I looked up the specs of Ballard fuel cells, Ballard is supposed to be a world leader in fuel cell technology, but their 75 kw fuel cell (granted it's designed for buses) weighs about 700 lbs, about x5 heavier than the higher power Honda design for a car!
Honda flow solution is run the two gases in channels vertically and the coolant runs horizontally across the vertical tubes. In its latest generation Honda has replaced the straight gas tubes with tubes that 'wave', claiming a 10% improvement as the gas flow is made turbulent. I am sure it also increases the cost of the fuel cell.
The US national energy lab has been working on this technology for a decade or so, ever since Bush announced the government would spend a billion dollars or so for hydrogen R&D. A 2011 NREL report looked at hydrogen car costs and estimated that at very high volume (1/2 million cars/yr) the cost of just the fuel cell (100 kw) could be brought down to $5,000 (don't know if this includes the platinum costs, which for 20 grams can be 1-2 k).
Honda and GM
In 2011 Honda announces that they will bring a hydrogen fuel cell car (Clarity) to market in 2015. In 2013 the Honda story changes to 'forget that shit'! The new Honda story is that they will begin working jointly with GM on hydrogen fuel cell technology, no doubt trying to get the costs down, and the new story headline is production cars by 2020. That tells you costs are now too high to go to market (tied up with this no doubt is the cost and time to develop hydrogen refuel stations), and pretty much insures that it will be seven more years before we see a production hydrogen fuel cell car from Honda or GM.
Mercedes BenzToyota Mirai --- 'production' hydrogen car
However, the story from Mercedes Benz appears to be just the opposite. Their news headline is that the date for their F-cell hydrogen car is hit the market has been brought forward from 2015 to 2014. Details in the story say Mercedes now has specific plans to manufacture quite a large number of hydrogen cars: 500 A type (for Europe) and 60 more advanced B type (for Germany and US). This is much more than any other manuf, but is it production or a large engineering run? It seems to be somewhere in the middle. The marketing dept may have decided to sell some of them, hence the 'production' claim. We will see next year. The interesting part will be the price.
Voltage control issueThe article says there are 260 hydrogen powered cars on the road in Germany vs 55,000 battery and plug-in hybrids. Hamburg is the focus of hydrogen powered vehicles in germany where a small network of refilling stations have been built. Daimler is planning to introduce a hydrogen powered SUV in 2017.
Toyota said that under unique driving conditions, such as if the accelerator pedal is depressed to the wide open throttle position after driving on a long descent while using cruise control, there was a possibility the output voltage generated by the fuel cell boost converter could exceed the maximum voltage.
Alstom the french manufacturer
has built a prototype hydrogen powered train engine that is undergoing
tests in germany. It says it has has preliminary orders for 50 to 60 of
these engines. Here refueling is much easier since a few refueling stations
can be built in railyards for trains that run on a schedule. 40% of germany's
local rail lines are not electrified (no overhead wires), so Alstom argues
to reduce CO2 emissions it would be cheaper to replace diesel engines with
hydrogen engines than to string electric wires.
------------------------
(6/25/17)
Toyota will now
sell you a Mirai for 57.5k, which includes three years of fuel free. It
features 5 min refills and 312 EPA miles per refill. It converts the electrical
output of the stack to 650 V allowing the Mirai to use the motor, PCU and
battery from various Toyota and Lexus hybrid cars. The battery helps accelerate
the car and provided regenerative braking. Toyota says they make the carbon
compressed hydrogen tanks themselves.
Toyota Mirai spec
fuel cell structure
Solid polymer electrolyte fuel cell (Proton Exchange
Membrane?)
number of cells in a stack
370
fuel cell power
114 kw
voltage booster
stack voltage boosed to 650 VDC (4 phase booster)
battery (hybrid)
xx kVA (Nickel Metal hydride)
electric motor
113 kw (pk, 3 PM phase synchronous)
motor torque
335 nm
hydrogen tank pressure
10,000 psi (70 Mpa, two tanks) (87.5 Mpa fill pressure)
hydrogen capacity
122 liters (5 kg approx) (60 L and 62 L tanks)
refill time
5 min (approx)
wheelbase
109.5 in
weight
4,078 lb (fuel cell stack -- 124 lb, hydrogen tanks (dry) 193
lb)
range
312 miles
EPA milage eq
68 mph
max speed
111 mph
acceleration
9 sec (0 to 60 mph)
----------------------------------------------------------------------------------------------
Toyota has
their 'production' hydrogen car on their web site. It has a firm base price:
57,500 and a new name: Toyota Mirai. Delivery date (in USA) is not so firm:
'coming in late 2015'. The car styling has also been tweaked so that
it really stand out (if in an odd way). Accompanying news articles indicate
there is less here than meets the eye. The world wide 'production' run
of this model is just 700 cars. However, the car does appear to be
real. Wikipedia says in one month (Dec 2014) it will to on sale in Japan,
then over 2015 will be made available in USA (sold only in LA where a few
hydrogen stations will be built) and in a few spots in Europe.
The car is built as a hybrid. It has a 1.6 kwh battery, which will power the car during low speed driving and which can be recharged by regenerative brakes.
The toyota web site shows the car uses two hydrogen tanks (different sizes). Range is given as 'up to' 300 miles and refuel time as 'in about five minutes'. Engine power is shown as 153 hp, equivalent to 113 kw from the fuel stack. From Wikipedia: curb weight is 4,078 lbs, wheelbase 109.4". The fuel cell stack is rated 3.0 Kw/L. Fuel is stored in two carbon fiber tanks at 70 Mpa, a short fat tank between the rear wheels and a longer thinner tank under the rear seat. At 4,078 lbs the car is fairly heavy, the fuel stack is reported to weigh 124 lbs and the fuel tanks (total) 193 lbs holding about 5 kg of hydrogen. Top speed is 111 mph and 0-60 mph takes 9 seconds. Fuel cell specs: 370 cells (single line stacking), 1.34 mm, 102 grams, 3d fine mesh channel, titanium separator, 114 kw (max output). The fuel cell output voltage is boosted to 650 VDC same as in many Toyota hybrids.

Toyota Mirai FCV 'production' hydrogen car (Nov 2014)
Coming late 2015, 700 car production run
(capture from Toyota video)
(2017 Toyota Mirai is a little restyled from this 2014 model)
Toyota FCV-R (2012)
Toyota is also planning to introduce a hydrogen car (FCV-R) in 2014 or 2015. There are pictures and it looks very stylish, but its spec have yet to be released. It will be shown at Tokyo auto show in Nov 2013, so more info will probably be available in a few months. It's rumored to cost between 50-100k (perhaps closer to 50k), have a 300 mile range, and could begin to be available in 2014.

(update 10/9/14)Fuel cell technology
Well, it's nearly a year since the last update on the Toyota FCV, so I did a search for news and (surprise!) there is very little. However, in a step forward in June 2014 Toyota did announce a US price: 69,000. (Price on a low production, new technology vechicle can be subsidized and observers suspect strongly that that is the case here.) Production date is slipping too, now spoken of a maybe end of 2015. It's a four seater, no picture of interior is avalable. Range will be about 435 miles. No detail spec has been released.(update 11/22/13)
Well Toyota did indeed show the FCV fuel cell car at the Tokyo Auto show Nov, but apparently little hard information was released. The NYT story about it said it will begin to be sold "around 2015" for a price that is undisclosed. The production car will look a lot like the show car (somewhat changed from above with different grill), but the show car is a 'concept' car. It will at first probably be sold only in CA and Scandavia to affluent buyers. The only hard information in the NYT story was the car's range: 310 miles.FCV info from Toyota (11/22/13)
On the Toyota web site some information about the upcoming production FCV fuel cell car is available.The most interesting is that Toyota is doing something different with the fuel cell stack than other manufacturers. Toyota Prius has long used a "boost converter" to step up and regulate the hybrid battery voltage from 300V (nom), which sags under acceleration, to 600VDC. They are going to use a boost converter in the FCV too, saying it allows them to reduce the number of fuel cells. No hint as to the voltage or number of cells. It is still a 100+ kw stack, so what this means in fewer cells each with larger area. So if they can reliably do larger area, higher current cells (no pin holes, or lateral thermal issues), then it should indeed help reduce the complexity of the stack and probably correspondingly its cost. And if the cooling is good within the stack, by regulating the voltage, they can be less concerned in the cell design by impedance that will cause voltage sag. It should open up the design parameters of the stack simplifying the design and probably lowering cost. This might be a good (incremental) step forward.
# of tanks 2
pressure 70 Mpa (10,000 lbs)
range 310 miles
refueling time 3 min
fuel stack power 100 kw ("at least")
boost converter yes
fuel cell location below seats
wheelbase 109.4"
seating 4Toyota also says they have halved the volume of the fuel cell stack (per kw) compared to their previous generation fuel cell.
Battery researchHyundai
Toyota says they are doing research on two new battery types, 'next generation' batteries, intended to replace lithium ion batteries.One is the lithium air battery. 'Air' type metal batteries cut the size of a battery roughly in half by replacing the cathode material with oxygen pulled from the atmosphere. Zinc air batteries work this way and are widely used. The classic problem with this type of battery is that the flow of oxygen is a choke point, so it tends to create batteries with low current (low power), but of course for cars what is needed is a high current battery.
The other is a lithium ion cell using a solid state electrolyte instead of a liquid electrolyte. The beauty of such a cell is how well it packages to make a high voltage battery. They show four cells stacked up within a single enclosure to made a 14.4V battery. This should made for a substantial reduction in size and weight of the battery.
First to market with a production hydrogen car might be Hyundai. The news stories are that between 2011 and 2015 they will build 1,000 (prototype) cars (ix35), billed as manufactured on a "production line" says Hyundai. 15 were delivered to Copenhagen in June 2015 for use by the city, where there is a single hydrogen refueling station. In 2015 an improved model is planned with a 10k production run.

Hyundai ix35 hydrogen fuel cell 4 door sedan
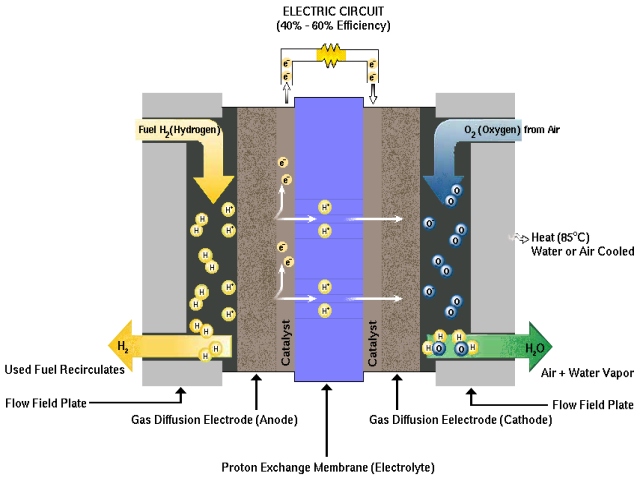
Anode: Hydrogen is oxidized (2H2 => 4H+ + 4e-)
Membrane: protons diffuse across
Cathode: Oxygen ((from air) is reduced (O2 + 4H+ +
4 e- => 2H2O + heat)
Here is a photo of the cross-section of the triple sandwich at the heart of each cell: [anode electrode (with platinum) + membrane (proton conducting) + cathode (with platinum)]. It shows this assembly is extremely thin (0.02 mm), which it needs to be because in a fuel cell stack all the current (333A nom @ 100 kw) must diffuse though 440 of these cells in series. (This may be thinner than what is going to used in cars, because this comes from ITM Power, who are doing membrane research to push up the current density. Yes, the standard PEM material is Nafion, and Dupont sells it in two thicknesses: 50 and 100 microns. This is still really thin a 1/20th and 1/10th of a mm.)
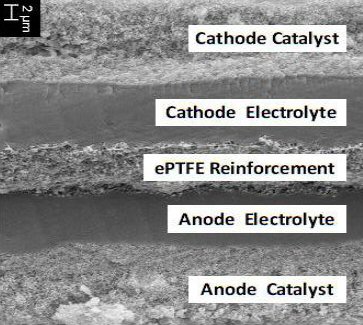
Cross-section photo of triple sandwich [membrane +
two electrodes] of an R&D cell
though which the current in the form of hydronium
ions (H3O+) must diffuse
shows it is only 20 microns (0.02 mm) thick
(Nafion, the baseline PEM material, comes in 50 and
100 microns thicknesses)
(source -- http://www.itm-power.com/wp-content/uploads/2012/04/HighPowerDensityFuelCells-Hannover-040411.pdf)
A first order approximation would assign all the efficiency loss of the cell to the voltage drop across the sandwich. At full power (100 kw, 333A) this would be 0.5V = (1.2 V ideal - 0.7V out), which means the power dissipation in each sandwich would be (333A x 0.5V) = 166W. My estimate from the cell I vs V plot is that the area of the sandwich is roughly 64 sq in, which makes the power loss 2.6 w/in^2 @ 333A. The power loss for the whole fuel cell at full power comes to an amazing 73 kw =(166 w/cell x 440 cells).
History
In 1839 Sir
William Grove doing an electrolysis experiment observed when the power
was off the current reversed. His two test tubes, one with hydrogen, the
other with oxygen, each platinum electrodes formed a gas battery with a
voltage of one volt.
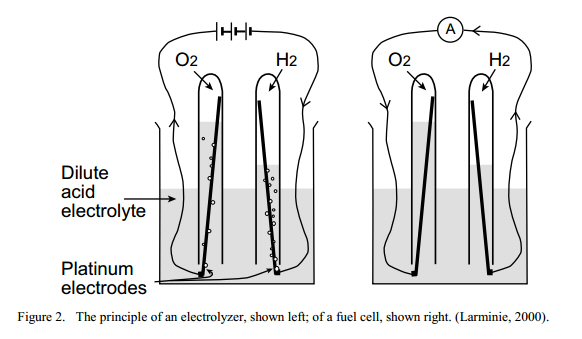
simple fuel cell, Voc = 1V (approx)
(source -- http://fuelcellstore.com/products/heliocentris/INTRO.pdf)
Role of platinum
catalysts
The membrane
material used in a PEM cell is a polymer. PEMs are generally produced in
large sheets. The electrode catalyst layer is applied to both sides, and
is cut to the appropriate size. A single PEM sheet is typically between
50 to 175 microns thick, or around the thickness of 2 to 7 sheets of paper.
This reference (http://fuelcellstore.com/products/heliocentris/INTRO.pdf) describes the reaction in some detail describing what the platinum catalyst does:
At
anode
-- The hydrogen molecules coming into contact with the platinum catalyst
on the anode electrode surface break apart,
bonding to the platinum surface forming weak H-Pt bonds.
-- The remaining hydrogen proton bonds with a water molecule on the membrane
surface, forming a hydronium ion (H3O+).
The H3O+ hydronium ions travels (diffuse) through the membrane material
to the cathode, leaving the platinum catalyst
site free for the next hydrogen molecule.
At
cathode
-- Oxygen molecules come into contact with a platinum catalyst on the cathode
electrode surface. The oxygen molecules
break apart bonding to the platinum surface forming weak O-Pt bonds.
-- Each oxygen atom then leaves the platinum catalyst site, combining with
two electrons and two protons to form one molecule
of water. The platinum catalyst on the cathode electrode is again free
for the next oxygen molecule to arrive.
Comments on the role of the catalysts
While I never
saw this explicitly stated, I think there is little doubt that the reason
catalysts are needed in fuel cells is to make low temperature (<
80C) hydrogen 'burning' go at a reasonable rate. It's like in our body.
We are only able to oxidized they hydrocarbons in food at a reasonable
rate at body temperature, because we have specialized enzymes to hold reacting
molecules in place. They do the same job as catalysts in a fuel cell.
a) I read that platinum is considered to be the perfect catalyst at the anode where it effectively splits H2 and strips off the electrons, but a really good catalyst for the cathode, which has to split O2 and assist H2O to form, has never been found. Platinum is again used here because it is the best known.
b) Note the above description has a continuous flow of water across the membrane from anode to cathode, since each protons is being carried across the membrane by glomming onto a water molecule. How does this work? There is no (outside) source of water at the anode only H2. The only way I see this working is by a counter flow of H2O developing within the membrane causing H2O molecules to diffuse (or flow) back from cathode to anode. The needed H2O molecule exists at the cathode. The water output from the cell can be considered to be the water molecules created when [O + 2H+ + 2e-] combine. As new H2O molecules are created at the cathode, the H2O molecules that carried over the needed H+ become plain old H2O again and are free to 'flow' back to the anode. (Does this just happen naturally? I can see how an H2O diffusion gradient might build up across the membrane as water molecules 'pile up' at the cathode. This would then be the source of the counter flow providing the anode with H2O molecules to pick up more H+. It does form a neat picture.)
c) The anode is the negative terminal and cathode the positive terminal. An E field exists across the membrane pointing from the cathode to the anode. Note this means the H3O+ hydronium ions are diffusing against the E field. The E field is not doing work on them, they are doing work on the E field. This I think is the source of the power out of each cell. (This is off the top of my head, but it seems right. When ions are pushed by an E field (for positive ions the push is in the direction of the E field), work is being done on the ions, the ions are absorbing energy and release it as heat when they collide.)
d) Saw a paper that argued the four electron reaction kinetics of making two H2O from O2 plus protons and electrons flowing in is tricky. The three incoming items (O2, protons, electrons) have to be briefly held in the correct position for the desired reaction to occur. This is why a cathode catalyst is needed in a fuel cell, to speed the desired reaction to water and to block competing reactions.
e) An important job of the catalyst on the cathode is insure that (most of) the output is water (H2O) not hydrogen peroxide (H2O2). I saw a forum posting asking why fuel cells produce water not hydrogen peroxide. After looking at the molecular structure of hydrogen peroxide, I think that is a good question. I haven't worked a bond energy calculation, but from its molecular structure hydrogen peroxide does look like a possible (or likely) output of a fuel cell. Hydrogen peroxide (H2O2) is two oxygen atoms attached by a single bond with hydrogens tying up 2nd bond on each oxygen. Since the incoming oxygen molecule (from the air) is two oxygen atoms double bonded, it looks like it would might be easier to make hydrogen peroxide than water, just break one of the two bonds between the oxygen atoms and attach a proton and electron to the free bond of each oxygen!
A canadian company, Ballard Power (http://www.ballard.com/), I read is the world leader in PEM fuel cells, and are working with many car companies.
What
is the heat value of hydrogen?
The starting
point for calculating the efficiency of a fuel cell is the 'heat value
of hydrogen'. Turns out the answer to this question is a little bit tricky,
and it took me a while to decode the jargon here. The 'heat value' is how
much energy is released when hydrogen is 'burned', i.e. it combines with
oxygen yielding heat and water. Hydrogen has what is called a 'low' and
'high' heat value, which differ by about 18%; the heat value of 1 kg of
hydrogen can be found as 120 Mj (low value) or 142 Mj (high value). Turns
out when you burn hydrogen for heat, say in a boiler, how much heat you
get depends on the design of the boiler. If the resulting steam goes out
the stack, you get close to the low value, if the steam is condensed (condensing
boiler) you get close to the high value.
The difference
between the high and low energy value is (mostly) due to the 'heat of vaporization'
of the reactant water. Water absorbs a lot of heat when it makes a phase
change from liquid to gas. It is clear that the total energy released by
the burning of hydrogen is the 'high value' (142 Mj per kg of H2). If the
water created escapes as vapor, then it should be viewed as an efficiency
loss, the water vapor carrying off about 15% of the energy the oxidation
reaction of hydrogen has produced (22Mj of 142 Mj per kg). And of course
hydrogen in a fuel cell is (in effect) being low temperature 'burned' in
a reaction speeded by catalysts, and it appears that (probably) most (or
all) of the water produced by a fuel cell comes out of a cell as water
vapor. So here is a 15% loss term in fuel cell, 15% of the potential energy
of hydrogen that will not be in the electrical output. Even if some of
the water vapor created in a fuel cell condenses before exiting the tail
pipe, it does not help the efficiency as it only creates heat.
-------------------------------------------------------
Efficiency
A fundamental
limit of fuel cells is that about half the heat energy in the fuel (in
practice more than half) is released not as electricity, but as
heat. This come directly from the chemical equation where the formation
of water from oxygen and hydrogen (opposite of electrolysis) is exothermic.
Wikipedia says theoretical efficiency of a fuel cell is 40-60%, but in
a typical vehicle driving cycle of fuel cell cars the 'tank to wheel' efficiency
is about 36%, and when the external loses required to compress the hydrogen
are included, this drops to 22% (and is even lower, 17%, if the hydrogen
is stored as a liquid).
Enthalpy of combustion for hydrogen is -286 kj/mol, so when one mole of H2 (2 grams) combines with oxygen, i.e. burns, 286 kj of energy are released as heat.
H2(g) + (1/2)O2(g) => H2O(l) + 286 kj
Thus the (combustion) energy potential of 1 kg of H2 = 142 Mj (eq 40 kwh) (142 Mj = 500 mol x 0.284 Mj/mol)
Round trip efficiency
A good way
to look at the efficiency of the hydrogen world is look at the energy (in
form of electricity) input in electrolysis to 'create' H2 and then at the
(total) energy released when the hydrogen is combusted with oxygen (from
air). References speak of the theoretical efficiency of electrolysis
(separation of liquid water into gaseous hydrogen and oxygen) as 100%.
I guess this means at a low enough rate where ohmic heat losses are negligible
all the electrical energy input is captured as potential (burn) energy
in the hydrogen (nope, it's not that simple).
The real world efficiency of electrolysis [output being the (combustion) energy potential of the hydrogen] is generally 50-80% and some of this loss is not ohmic, but is associated with 'over potentials' required to get the reactions to go. Over potentials can be reduced by catalysts (to zero?), but the best catalyst is platinum, which is of course very expensive. With platinum catalysts electrolysis efficiencies up to 82% have been reported.
Clearly when H2 is combusted in a fuel cell the released energy is split between (usable) electrical energy and (waste) heat energy. I can't find any theoretical (upper) limit on this split, it just seems to depend on engineering of the cell. While Wikipedia ('Fuel cells') says the general efficiency is 40-60%, in a table PEM fuel cells (proton diffusion types used with hydrogen in cars) are shown as 50-70% efficient.
Waste heat
So if fuel
cells are only 50-70% efficient is getting rid of the waste heat a problem?
Probably not. They might dump much of their waste heat the same way a normal
internal combustion engine does, it's carried away by the output gas, here
steam (H2O) that goes out the tailpipe. Some of the waste heat is used
to raise the operating temperature, which is typically 80C. However, these
cars also have a coolant system quite similar to normal car. Coolant (anti-freeze
+ water) is pumped through the fuel cell and then to a large radiator behind
the grill.
Hydrogen vs electric
Unlike electric
cars which inherently capture deceleration energy of the car pumping
power back into the battery, when a hydrogen fuel cell car slows down and
the DC bus voltage pumps up, the fuel cell does not run backwards
and pump hydrogen back into the tank!
Ignoring price (and emissions) prototype fuel cell hydrogen cars have demonstrated a huge advantage over electric cars in that they have more range and can be refueled quickly. In other words there are 'real' cars, or as an US energy lab guy said, they are a 'direct replacement for any vehicle'. But a HUGE drawback of fuel cells cars is that they look to be far too expensive to build, plus they use lots of energy to run (with all the CO2 emission that implies) because the [electricity => hydrogen => electricity] process is fundamentally inefficient.
Cell V vs I
I did an image search
and turned up this interesting graph of voltage vs current for a single
cell. Don't know whose technology this is, so the numbers may be off, but
the general characteristic of how the voltage varies with current is very
interesting, but since I think it comes directly from the electrochemistry,
it is probably representative of all hydrogen 'burning' fuel cells.
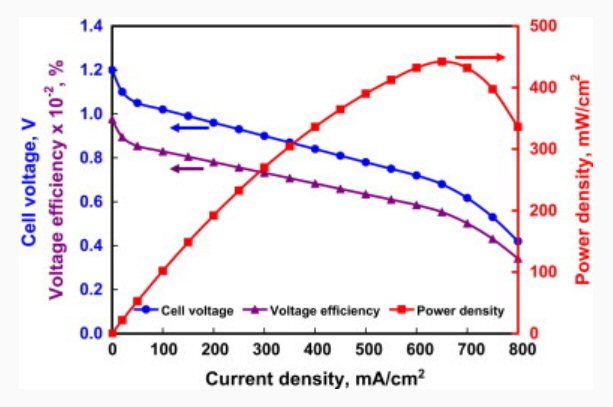
blue -- voltage vs current for a single cell of a
hydrogen fuel cell
red -- plot of cell power (V x I) vs I
[operating current density: 0.65A/cm^2 @ 0.7V/cell]
(source -- http://origin-ars.els-cdn.com/content/image/1-s2.0-S0360128512000044-gr3.jpg)
The voltage curve is very interesting. Note that at zero current the voltage (Voc) is 1.20V, this is almost exactly the ideal voltage of electrolysis of water (1.23V) into H2 and O2 gas. A hydrogen fuel cell has the same (ideal) equation, since it is the same reaction in reverse, H2 and O2 combine to make water. The electrochemical potentials of the half reactions show: 0V at the anode where [H2 => 2H+ + 2e-] the electrons being split off by the platinum catalyst, and 1.23V at the cathode where [2H+ + 2 e- + 1/2 O2 => H2O].
As the cell is loaded and begins to put out electrical power, the voltage sags. At the maximum power point (peak of the red curve), where a fuel cell would be designed to operate at maximum power, the output voltage is down to 0.7V. Now this agrees with the references, but it also allows us to estimate the efficiency of the cell, i.e. the split between useable electrical power and waste heat. The estimate comes from dividing the output voltage (at max power) by the output voltage at zero power. When we do this, we get [0.7V/1.2V = 0.58], which hints strongly that at maximum power the cell is only 58% efficient. A simple battery type model, which might not exactly apply to fuel cells, tells us at high current 42% of the potential cell voltage is dropping internally across various aspects of the cell, so [I x (1.2V - 0.7V] is the power of the reaction that produces heat in the cell. This power is substantial, it is why fuel cells need to be cooled. When the car's fuel cell stack is outputting 100 kw of usable electrical power, it is at the same time generating [(0.42/0.58) x 100 kw = 72 kw] of waste heat. Some of this heat come out with the output water, the rest would have to be flushed out by the antifreeze and brought over to the radiator.
We can get an additional piece of data from this plot, an estimate of the area of each cell in an auto fuel cell. The Honda spec says there are 440 cells inside and since [440 cells x 0.7V/cell = 308V] this almost for sure tells us that there is no paralleling, all 440 cells are in series. Using round numbers for 100 kw fuel cell [300VDC x 333A] tells us 333A has to pass through every cell at maximum power output. The above curve shows that for this (unknown) cell design the power is maximum at a current density 0.65A/cm^2. This would mean the cell area of a 100 kw series stack would need to be (300A/0.65A x 1 cm^2 = 462 cm^2) or 8.5 " x 8.5" (72 in^2). This is a reasonable number since it looks consistent with the size of the Honda fuel cell package. My eyeball estimate is that four stacks of 110 cells each of this area would fit inside, and to keep the stack length to about a meter the thickness of each cell would need to be about 1/3 inch.
We can push this analysis a little further. At 333A x 0.5V (internal) drop each cell is dissipating 166 watts. I read that one of the tricky engineering challenges in fuel cell design is managing lateral instability of the cell heat, or in plain language cooling the cell evenly and preventing hot spots from developing that will damage it. (While no details were given, the implication of the reference I found was that there is (or might be) a thermal instability, which typically means when an area gets hotter the voltage goes down so current tends to crowd there, which makes it even hotter, etc.)
Another i vs v plot
I later found another
(i vs v) plot from a UK company, ITM Power who have worked for years on
increasing the power density of the membrane. Same general shape and voltage
as previous plot, but for this membrane the current density is higher (1.5A/cm^2
@ 0.7V) and higher still at lower voltages. Since this is an R&D membrane
designed for higher power density, it roughly confirms that 0.65A/cm^2
@ 0.7V/cell is probably in the ballpark for the current density in fuel
cells now in cars.
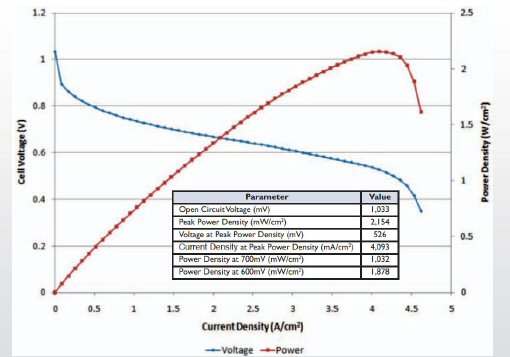
fuel cell V vs I from ITM Power (in development)
1.5 A/cm^2 @ 0.7V/cell (3A/cm^2 @ 0.6V/cell)
(source -- http://www.itm-power.com/wp-content/uploads/2012/04/HighPowerDensityFuelCells-Hannover-040411.pdf)
My PEM membrane
model
I think I
understand pretty well how the membrane (PEM) at the heart of a fuel cell
works. It is a very thin (1/10th mm) solid material, typically Nafion,
invented over 40 year ago for use in fuel cells for the space program.
It absorbs water (it 'wets') allowing it to work as an electrolyte for
hydrogen fuel cell. The H+ ion has a natural affinity to glom onto a water
molecule (forming the hydronium H3O+ ion), and the material is designed
to allow water molecules to move around. A voltage across the membrane
will drive the H3O+ ion (with its H+ load) across the membrane, so the
membrane can 'conduct protons'. The voltage across the membrane (see figure
below) is a fraction of the voltage that develops at the cathode (1.23V)
when hydrogen, in the form of H+ and e-, is oxidized by O2 (from the air)
to make water. To make a battery the membrane needs another property, it
has be impervious to the diffusion of H2 and O2 to prevent uncontrolled
oxidization, sometimes called a 'gas short'.
It is clear that proton conduction across this type of PEM membrane depends on internal water flows inside the membrane. This leads to one of the limitation of current fuel cells, they cannot be run above 80-90C otherwise the membrane would dry out and stop working. This is a serious limitation as a fuel cell is also a heat engine and thermodynamically it would run better if it ran hotter, also I read catalysts are able to clear themselves better at higher temperature. There is also a corresponding low temperature problem that affects cold starting, but engineering work here has paid off with prototype hydrogen cars rated to start at temperatures well below 0F.
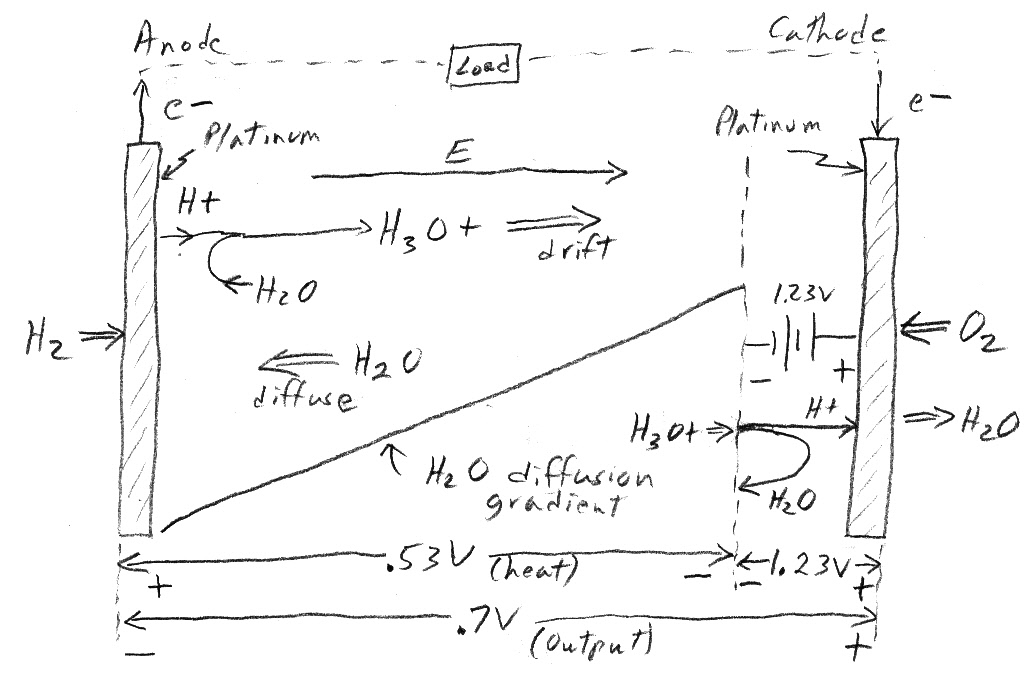
my PEM model sketch (9/8/13)
Positive hydronium ion (H3O+) is 'pulled' (right)
toward cathode by a negative voltage (0.53V),
which is a fraction of the cathode reaction battery
(1.23V).
An H2O diffusion gradient automatically sets up and
returns the freed up water to the anode.
Power generation is indicated by the (positive) ionic
current flowing into negative terminal of the cathode reaction battery
(1.23V).
Heat is generated by the (positive) ionic current
flowing down an electrical gradient (0.53V) across the PEM.
The difference voltage [0.7V = 1.23V - 0.53V]
is available at the external terminals to do useful work.
I have not been able to find a battery type model for the inside of the PEM membrane, but from text descriptions of how it works and my understanding of batteries I have drawn it up (see above). I think it explains all the key aspects of the fuel cell 'battery'. The reaction at the cathode as the H+ (plus returning electrons) drop into the electron orbit holes of oxygen making H2O can be modeled as a 1.23V battery (ideal). This battery is drawn at the cathode (positive terminal), which is where the reaction occurs. Under load the cell voltage can be measured to be about 0.7V. This means that as seen from inside the membrane the cathode (positive battery terminal) is seen to be about a half volt negative with respect to the anode (negative terminal), thus an E field across the membrane develops pointing from the anode to the cathode.
This E field drives the hydronium ions (H3O+ = H+ + H2O) across the PEM (left to right). Since H+ ions coming in from the anode 'stick' to water molecules forming hydronium ions (H3O+), water molecules are also being driven by the electric field (left to right) to the cathode. The pile up of H2O near the cathode is self correction because an H2O diffusion gradient then builds up until a balancing counter flow of water molecules (right to left) back to the anode exists. So under load a water loop automatically forms within the PEM that returns water molecules to the anode after each drops off its H+ load at the cathode.
This model also allows the efficiency of the fuel cell to be calculated from the voltages. The model shows 0.53V of the 1.23V from the cathode reaction appears across the PEM driving the protons across the membrane. This voltage times the current represents a heat loss within the membrane (in a real cell the heat is more spread out) as the positive ionic current flows downhill thermodynamically. The remaining 0.7V of the electro-chemical 1.23V from the oxidation of hydrogen (reverse of water electrolysis) appears at the external terminals available to do useful work. Hence the fuel cell efficiency shown by this model is 57% (0.57 = 0.7V/1.23V), which is known to be in the right ballpark.
The heat generated in each cell is considerable at full load (300A x 0.5V) = 150 watts. Since there are 440 cells (typ) in a hydrogen car fuel cell, this is 66 kw of heat (440 x 150 watt = 66kw) that the coolant needs to dump, assisted by the heat the hot H2O exiting the cells carries off out the tailpipe.
How are cells
stacked?
This figure
shows the concept of how cells are put in series. A series stack up requires
the gases running down the stack alternate (hydrogen, air, hydrogen, air,
etc). The key to doing this is the electrode (below) in the middle. It
has channels on both sides, one side for air (oxygen) and the other side
for hydrogen. If all the electrodes are made like this, a series stack
can be built. Clearly a requirement is that the electrode material (thick
black) be electrically conductive by not allow gases to diffuse through
it. The triple sandwich between composed of the thin anode material, membrane
(green), and thin cathode material must all allow hydrogen/proton diffusion.

Terminal with double sided gas channels (H2 one side,
air other side)
allows fuel cells to stack in series
There's a complication which this figure does not explain well. The molecule picture is that electrons are released on the thin anode material (thin black) and electrons combine (with H+ and O2) on the thin cathode material (also thin black). If an external terminal is to be attached, it goes on the much thicker electrode (black with gas channels cut in it). For this all to work it must mean that electrically the cathode, electrode, and anode are all shorted together, which would happen here if you visualize everything squeezed together. It shows there is a complication in the design, one the one hand you want a low impedance path from the anode and cathode to the terminal, but some of the area can't be used (maybe half) because it is needed for gas flow channels.
Bipolar platesMembrane issues
The fuel cell literature uses the term 'bipolar plate' to refer to the center terminal above (the thick black one with hydrogen channels on one side and air channels on the other side). This is one of the most expensive parts of a fuel cell as it has lots of jobs: conduct current and heat, be strong and light, yet have complex channels on both sides and be impervious to gas diffusion. According to the (detailed) 2011 fuel cell reference below, how to manufacture it in volume is an unsolved problem. It is now made of graphite.Ref: http://electrochem.cwru.edu/encycl/art-f04-fuel-cells-pem.htm
A basic characteristic (and limitation) of it is it must be kept 'wet' (hydrated), but not too wet. Not so easy in a cycling machine that must run over a wide range of temperatures and humidifies. Protons are carried across the membrane by glomming onto water molecules, so 'wet' is not a detail, it is how it works. This limits the internal temperature of the fuel cell, it must stay below 100C. It limits cold temperature starting too, because it freezes up, though manuf here must have some tricks (added anti-freeze?) because the new fuel cells cars can start down to -13F. It cannot be allowed to flood, so the water being continuously created must be effectively carried away. I read that allowing the operating temperature of the cell to rise to 100C would make the cell less sensitive to impurities in the hydrogen fuel.
Misc issues
When you look
carefully at the 'how it works' fuel cell diagrams, there are hints a hydrogen
car require support systems rarely mentioned, and of course, the modern
combustion engine is now full of these systems, many for pollution control.
For example, a humidifier. In a technical reference on fuel cells (linked
by a hydrogen car association) the gas flow diagram shows a humidifier
(requires a water tank) located in the gas flow path between the hydrogen
tank and the fuel cell (and there was a dehumidifier too). Presumably this
is part of a water control subsystem needed to control the moisture of
the PEM membrane.
Simple 'how it works' diagrams nearly always show an exit path for the hydrogen, sometimes labeled 'unburned hydrogen'. Since the exiting hydrogen can't be returned the high pressure hydrogen tank, there must be some sort of recycle loop to merge it with the incoming hydrogen from the tank. I have never seen a single reference hint at what fraction of the hydrogen entering the fuel cell exits without being burned, or show how this works.
High pressure
hydrogen tanks
I found that
all the cars were storing hydrogen at either 5,000 psi or 10,000 psi. I
now know why. The tanks used all meet detail standards (for design, test,
and safety) for high pressure hydrogen storage from several regulatory
bodies. A small group, who I found recently had a conference devoted just
to this subject in China, has been has been worked closely with manufacturers
on these standards for 20 years! There are four classes of hydrogen tanks
(I, II, III, IV). I and II are metal based tanks and operate at 2-3
thousand psi. (A difficulty with metal is that it is made brittle by hydrogen.)
Newer III and IV tanks replaced the metal with carbon fiber and glass fiber
and pressure has been raised to 5,000 and 10,000 psi. (Type IV tanks were
first demonstrated in 2001.) These are the tanks being used in the cars.
Manufacturers of hydrogen cars don't need to design the tanks, the
tanks specs are now standardized, they just order a class IV (or maybe
class III) tank of the appropriate volume. The tank burst pressure is required
to be something like x2.25 the maximum operating pressure.
Tank volumeWhich tank class?
Motor trend magazine gives this data about compressed hydrogen:1 kg of H2 @ 10,000 <=> 1 cu ft (vol) (0.5 cu ft for liquid hydrogen)
Obviously a 240 mile range 4 kg class IV (10,000 psi) tank needs an interior volume of 4 cu ft. Surprisingly the volume of liquid hydrogn (at 20 degrees kelvin) is only a factor of two lower than at 10,000 psi. (For reference a gasoline tank of 4 cu ft would hold 30 gallons.)
Why only 4 kg of hydrogen?
10,000 psi
seems to be the technological limit for vehicle hydrogen tanks, at least
for the foreseeable future. I don't even see mention of a class V tank
in development. This means that the only way hydrogen cars have of increasing
their range from their current 240 miles (4 kg of hydrogen, nom)
is more tank volume. The early tank studies always (reasonably) assumed
that a hydrogen car would need to carry 6 to 7 kg of hydrogen for a 400
miles range. So what happened? How come the hydrogen car prototypes from
every manufacturer only have 4 kg tanks (nom), which gives them only a
range of 200 to 240 miles? Aren't they pissing away one of the biggest
(potential) advantages that hydrogen cars have over electric cars, long
range and fast refill?
It must be that they have all decided that to sell a hydrogen car must look like a 'real' car. What, they don't want to fill the trunk with tanks (or put them on the roof), to have the car look like a geekmobile? Nope, the tanks must be (pretty much) hidden. The one or two tanks in every car are under the floor at the back above the rear axle, generally surrounded by a metal frame for support and safety. It is imperative that the tanks be shielded from being cut, nicked or crushed due to road hazards or accidents. There is no choice of shape. The high pressure requires that large tanks have a cylinder shape with rounded ends.
Bottom line --- This (partially self imposed) tank limit of 4 kg of hydrogen (nom) makes the range of hydrogen cars marginal. Hydrogen cars in their early development were expected to carry 6-7 kg of hydrogen giving them a good 400 mile range, far above electric cars. But the Tesla model S electric car with its claimed 300 mile is a game changer. It has a usable range equal to, if not a little higher, than all the hydrogen cars coming to market. It could be argued that the car companies by being too conservative in their designs have pissed away what should have been one of the big strengths of a hydrogen car: good range. I mean the hydrogen fuel weighs essentially nothing. I can't find the weight of the tanks, but they sure weight a lot less than a large battery pack (maybe 1/5th to 1/10th). For example, why isn't there a customer option to add another tank to extend the range by 50%? Tesla Model S offers three sizes of battery.
Whoops, not all hydrogen cars are 4 kg, 240 mile range
When I got more car specs I found that two higher range models have been built in recent years. One is from Hyundai, i-Blue (370 miles, 6 kg est). It fits without apparent problem (see Hyundai chassis above) two 10,000 psi tanks in bottom rear. One is tank is bigger than the other, so it's looks like it might have been designed as one tank for the base model (240 mile range) and the second tank (possibly optional) adding 50% more capacity to give 370 mile range. The car with the highest range of all (516 miles, 8+ kg est) is from Toyota (below). Whether the coming production cars from Hyundai and Toyota will continue to have extra large tanks and high capacity only time will tell.
Tank wallsHighest range car
Toyota FCHV-adv (2008) mid size crossover apparently has the most range of the current generation of hydrogen cars. In the DOE report its range is shown as 516 miles and its class IV (10,000 psi) tank(s) have a capacity of 156 liter. This is 35% more tank volume than the Hyundai i-Blue spec above (156L/115L) which fits nicely with its 39% higher range (516 miles/370 miles). This Toyota must be carrying about 8+ kg of hydrogen to get its 500+ mile range vs about 6 kg for the Hyundai.
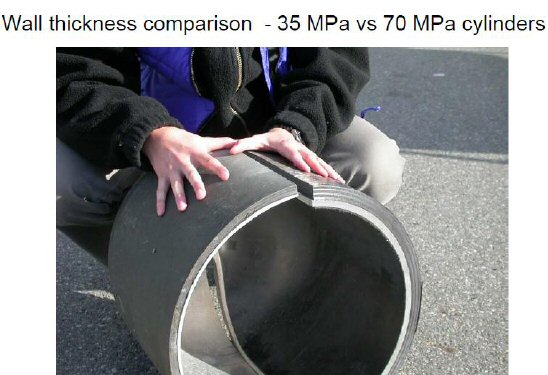
(source -- http://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/cng_h2_workshop_8_wong.pdf)
A defective or damaged high pressure tank can indeed explode like a bomb and will destroy the vehicle. The reference above has pictures of a bunch of vehicles that were accidentally blown to bits by a tank that let go!
Hydrogen density vs pressure
Here's an
interesting plot showing hydrogen density vs pressure. The ideal gas law
[PV = nRT] would say that doubling pressure from 5,000 psi to 10,000 psi
would double the density, but that's not exactly true at these high pressures.
Note the green line (300k, room temp) is not straight, it rolls off a little.
Values from a table show that doubling the pressure [5,000 => 10,000 psi]
increases the kg of hydrogen in the tank by x1.69, or about 15% less than
the ideal gas law would predict. This explains why some of my tank kg calculations
were off.
5,000 psi (1.43 lb/ft^3)
10,000 psi (2.415 lb/ft^3)
(15% less than the ideal gas law would predict)
hydrogen density vs pressure
(source -- http://www.inference.phy.cam.ac.uk/sustainable/refs/hydrogen/tank/28890m.pdf)
Tank cost
A recent gov
report says the estimated cost of a (current technology) class IV, 10,000
psi hydrogen tank at (very) high volume is $15.6/kwh. (In the case
of fuel cells the gov definition of 'high volume' is 500,000 units/yr,
so these are ridiculous high volumes.) The 4 kg (nom) capacity tank(s)
used in most of the cars is equivalent to about 160 kwh. The GM tank is
168 kwh, so ($15.6/kwh x 168 kwh) = 2.6k, so these high pressure
tanks are expensive! My guess based on this is that at more reasonable
volumes for the next few years the cost of a class IV tank(s) for 4 kg
(240 mile range) might be 4-5 k! It's might be nearly comparable in cost
to the fuel cell. (I found two refs from a few years ago that a class IV
tank this size cost 7k and 10k, but this was the early days of class IV
tanks and no quantity was specified, so the cost is pretty meaningless.)
Tank weight
Most of the
cost of class IV tanks is the raw cost of the carbon fiber. A footnote
in a report gave the amount of carbon fiber in a typ 4 kg car size class
IV tank as 47 kg and said this was 68% of the weight of the tank. This
translates into a tank weight of 150 lbs, so tank weight is not a problem
with high pressure class IV (metal free) tanks, it is the cost and volume.
Tank limit on fast refueling
There a problem
with fast refueling that is barely mentioned. When any gas is compressed
it gets hot, that can be felt even at only small compression ratios when
filling a bicycle tire. Hydrogen in class IV tanks, which most cars are
using, is HUGELY compressed by a factor of 680 = (10,000 psi/14.7
psi)! (How much of that compression occurs in the tank of the car
is hard to find out, but my guess is most of it.) I read one government
report that commented that the temperature rise in the tank caused by fast
refueling was an extreme problem.
Some (or all) of difficulty is connected to the design of the class IV tanks. They are not made of metal (there is no metal in them), they are composed of carbon fiber and epoxy. They can't stand high temperatures, the epoxy softens and looses strength, they are low temperature tanks.
Are refueling stations precooling the hydrogen?
The work around
discussed in early reports is precooling the gas. The refueling
stations would have to have a refrigerator system to cool the gas in storage,
so that the hydrogen entering the tanks is cold. This is known to work,
but it takes energy, it is another efficiency loss in the hydrogen system,
not to mention the added cost to build refueling stations. What are the
numbers, cool by how much? (For example, cooling by 50C would allow a transient
50C rise in the tank due to compression.)
SAE J2601 specifies precoolingHow the hell does refueling work?
Hydrogen refueling standards are set by the Society of Automotive Engineers (SAE) J2601 standard. I tracked down a set of graphs about the standard and it looks like precooling is used 10,000 psi (700 bar) tanks. 700 bar has two classes and both specify the hydrogen from a filling station be precooled (-20C and -40C as I remember).Yes, Air Products data sheet on their hydrogren fueling pump says it complies with SAE J2601 and the hydrogen gas is 'temperature controlled to -40C'.
No trick
As best as I can tell the hose and connection to the car must handle the full 10,000 psi. Somewhat hard to believe because the hose does not look particularly thick or strong (see photo below). The subject barely comes up on the sites of the two major manufacturers of hydrogen fueling stations, but both Linde and Air Products say the 'dispensing' pressure is 700 bar (10,000 psi).

The link below is a video made by Air Products shows a hydrogen car being refueled at one of their stations. I think this is an older video, since it looks like they are using the thinner 5,000 psi hose. (Refueling shown here is cludgy since beside the hydrogen pipe for the gas an electrical cable must also must be plugged in. It feeds back tank temp and pressure to the pump. I don't know how common this will be. The J2601 standard supported options on feedback.)
http://www.airproducts.com/products/Gases/Hydrogen.aspx
Linde refueling stations
Tracked down
a major manufacturer of hydrogen fueling stations: Linde
Group (German). They claim to be the world leading hydrogen refueler
having built 70 stations in 15 countries including in Tokyo and the only
hydrogen refueling station in SF, and working with Daimler they have plans
to build 20 hydrogen stations spread all over Germany. It looks like the
Germans are serious about building a hydrogen car car and infrastructure.
(A US company that build hydrogen refueling stations is 'Air Products',
and they also claim to have built many stations.)
Linde's specs say 'Dispensing Lines -- 700 bar -FCEV'. Which I guess means the hoses are the car/hose interconnect are carrying the full pressure of 10,000 psi! I find this quite remarkable. What kind of technology does this take? The car tank has 1+" of wrapped carbon fiber to hold this pressure, how do they do this in a flexible hose?? No one (not Linde) mentions it. Puzzled, but at least it explains how the fuel gets into the car tank at 10,000 psi (700 bar). (For reference I read heavy metal cylinders of various compressed gas are usually at a pressure of 150 bar, about five times less than the hydrogen car tanks.)
Could not figure out from the Linde site if the hydrogen gas for cars is precooled. Linde makes two types of pump/compressors for hydrogen refueling labeled 'ionic compressor' and 'cryogenic pump'. The cryo pump is a lot bigger with four times the flow (120 kr/hr vs 36 kg/hr, both rated to 900 bar). There are hints that that the cryo unit may precool, but no numbers. Linde indicates the bigger cryo unit is designed for large hydrogen gas stations as it has the flow rate to support several pumps (for use by 'public/bus operator'). Presumably the small (non cooling) compressor is used when a single hydrogen pumping is added to a conventional gas station, which is quite common in CA. But the numbers don't quite add up with the smaller pump. It's flow rate is 36 kg/hour, which means to refuel a 4 kg car (empty tank) it takes 6.66 mins, yet the car vendors all say it takes 3-5 mins to refuel. Who's lying?
Linde site
http://www.whec2012.com/wp-content/uploads/2012/07/NEW-ASR14.1.pdf
References
A lot hydrogen car specs (somewhat old):
I stumbled
onto a DOE crash test report on hydrogen vehicles and it has a table summarizing
the power train of almost all hydrogen cars.
http://www.nhtsa.gov/DOT/NHTSA/NVS/Crashworthiness/Alternative%20Energy%20Vehicle%20Systems%20Safety%20Research/811267.pdf
Online book on fuel cell technology:
http://brucelin.ca/scooters/pdf/ch3c.pdf
======================================================================================
PEM membranse are robust
Turns out
that the PEM membranes at the heart of a fuel cell are quite robust. They
have been around for a long time and can do a lot of jobs. I see on Wikipedia
that they are used in caustic cells that manufacturer a sodium chemical
on a large scale. I stumbled on development work that Siemens is doing
on how to use hydrogen to smooth wind turbine power, and their (apparent)
first choice for manufacturing hydrogen on a large scale from 'excess'
wind turbine power is a PEM based cell (below). In principle this is remarkably
like a car fuel cell, except it is run backwards. Water goes in, electrical
power from a wind turbine rips the water molecule apart by 'tearing off'
its two bonding hydrogen electrons, and then the PEM in a cell, by virtue
of the fact that it will conduct only protons, is able to separate the
hydrogen and oxygen atoms into their respective gases. Electrolysis on
a large scale.
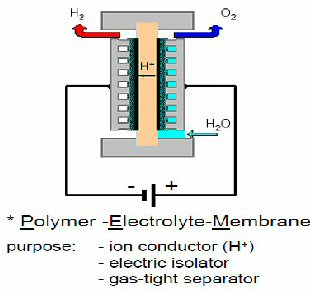
Siemens (proposed industrial scale) PEM electrolysis
cell
Right: H2O => 2H+ (through membrane) + 2e- (out to
power source) + (1/2) O2 (gas)
Left: 2e- (from power source) + 2H+ (from membrane)
=> H2 (gas)
(source -- http://www.dwv-info.de/aktuelles/Pressemeldungen/2012/pm1201_wai.pdf)
In a slide talk about this technology Siemens gives these interesting numbers:
9 liters of water yield 1 kg of hydrogen
50 kwh (approx) electrical energy generates 1 kg of hydrogen
1 kg of hydrogen contains 33.3 kwh of energy (33.3
kwh x 3,600 sec/hr = 120 Mj, 'low' energy value of hydrogen)
When I thought about it, the 9 liters is easily explained. Basic chemistry tells us 1 ml of water has a volume of 1 cm^3 and weighs 1 gram, hence 9 liters of water weighs 9 kg. H2O by atomic weight is 2 parts hydrogen and 16 parts oxygen, so clearly 9 liters (9 kg) of water when its atoms are separated will produce 1 kg of hydrogen gas and 8 kg of oxygen gas.
Electrolysis efficiency
Siemens says to
make 1 kg of hydrogen takes 50 kwh (eq 50 kw for 1 hr) into the PEM cells.
The resulting energy value of manufactured 1 kg of hydrogen is 33.3 kwh
says Siemens, which of course allows the efficiency of the process to be
calculated. Siemens numbers indicate that only 66.6% (.666 =33.3 kwh/50
kwh) of the input energy ends up in the hydrogen.
Low energy value of hydrogenWe can also use these numbers to estimate the cost to make a tank full of hydrogen. If car range is 360 miles (6 kg of hydrogen) and electricity cost 12 cents/kwh (USA average), then [6 kg x 50 kwh/kg x 0.12 dollar/kwh = 36 dollars], not cheap, but less than gasoline. It cost me with gas about $4/gal about 36 dollars for half a tank of gas.
Curiously the number Siemens gives for the energy value for a kg of hydrogen (33.3 kwh) calculates out to be 120 Mj, which is the 'low' energy value of hydrogen. It does not include the energy that went into vaporizing the 9 kg of water, in effect assuming this energy is unrecoverable, which I think is right when hydrogen is run through a fuel cell to create electricity.However, the consequence of assuming a 'low' value for the energy in hydrogen produced is to make the efficiency of its electrolysis cell look poor (66.6%). If the 'high' value of hydrogen had been used, the energy in the hydrogen would be higher by (142 Mj/120 Mj = 1.18), so they could quote the efficiency of their electrolysis cell as 79% = (1.18 x .666). (It makes no difference to the efficiency of the hydrogen cycle, it's just a question of accounting, whether to assign the water 'heat of vaporization' loss to the electrolysis cell or the fuel cell.)
'High' and 'low' hydrogen heat value
When water
vapor is the output of an exothermic reaction, there are two ways to figure
the energy 'released' (as heat), and they differ by 18%. "The higher
heating value of hydrogen is 18% above its lower heating value (142
MJ/kg vs. 120 MJ/kg). (Wikipedia, Heat of Combustion). I was initially
confused by this, but it is now clear.
It's a simple matter for accounting for the energy that goes into making output water a vapor, which for water is approx its heat of vaporization (40.7 kJ/mol) or 2.26 MJ/kg (of water).
How PEM electrolysis cell works"Most applications that burn fuel produce water vapour, which is unused and thus wastes its heat content. In such applications, the lower heating value is generally used to give a 'benchmark' for the process; however, for true energy calculations the higher heating value is correct. This is particularly relevant for natural gas, whose high hydrogen content produces much water. The gross energy value is relevant for gas burned in condensing boilers and power plants with flue-gas condensation that condense the water vapour produced by combustion, recovering heat which would otherwise be wasted." (Wikipedia, Heat of Combustion)
I-V curve
Siemens gives the
curve (below) for their PEM electrolysis cell. I think the "alkaline" reference
is that in practical cells some easily ionized material (acid) must be
added to the water. The reason for this is that pure water has very few
ions, so it conducts electricity very poorly. For the reaction to go at
a good rate the resistance of the water needs to be drastically lowered
so lots of current can get to the water molecules at the surface of the
catalyst without generating a lot of heat.
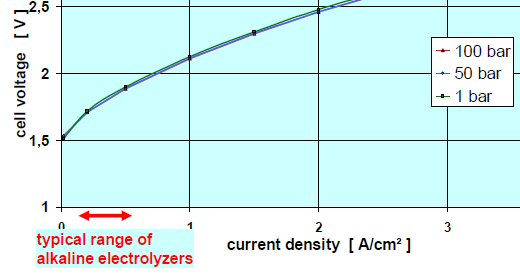
(source -- http://www.dwv-info.de/aktuelles/Pressemeldungen/2012/pm1201_wai.pdf)
The necessity to ionize the water is where this PEM electrolysis cell differs from the car PEM fuel cell. In a car fuel cell no electricity passes through the water, so its resistance doesn't matter. In fact pure is good ('No Pollution'). In the early space program PEM fuel cells astronauts were able to drink the water the fuel cells put out.
We can check the voltage in the curve against the Siemens efficiency number. A 66.6% efficiency would imply that the electrolysis voltage for water (1.23V, ideal) divided by the actual cell voltage would equal 0.666, or put another way the actual cell voltage when running at rated load will be 150% of the ideal voltage. This calculates to a cell voltage of 1.85V [1.23V/1.85V = 0.666], which is right in the 'typical range' marked in red on the curve.
Siemens electrolyzer
The Germans
have already installed a lot of wind (and solar) power with plans to build
much more. Reportedly 20% of the (potential) wind power is now being wasted
at night since the load is low. They have the space to store huge amounts
of hydrogen. An article in MIT magazine said they are taking seriously
the option of using hydrogen on an industrial scale to smooth out the power
from renewables. It may work, but the round trip efficiency is BAD, about
40% of the energy is lost in each conversion, so only about 1/3rd of the
original energy is recovered. Still it can be argued that it is better
than just feathering the turbine blades and wasting the 'free' power.
Siemens has
been working on an electrolyzer for this job and has developed a PEM electrolysis
system that is robust and can work on a large scale with surging currents.
Their PEM electrolysis cell is interesting, because (at least in principle),
it's basically the same as the PEM fuel cell except the current
is run backwards and water is fed in and hydrogen and oxygen (of high purity)
come out.
-------------------------------
High and low heat values
Difference
between high and low value for exothermic reactions. The energy of reaction
products can be taken as hot (actual) or they can assumed to be brought
down to the original (room temperature). For water this means basically
accounting for the heat of vaporization. In other words if reaction yields
water vapor, then some of the heat released by the reaction has gone into
vaporizing the water, so by one set of accounting it is not 'released'
because it is 'trapped' in the material. If water vapor is condensed, which
happens in some processes than the heat of vaporization is released and
comes out externally.
" Lower heating value (LHV) (net calorific value (NCV) or lower calorific value (LCV)) is determined by subtracting the heat of vaporization of the water vapor from the higher heating value. This treats any H2O formed as a vapor. The energy required to vaporize the water therefore is not released as heat." It's all just accounting. If you have a hot reaction product how do you account for the heat it has absorbed.High and Low voltage values
Low
value
(120 Mj/kg) x (1/500) for 1 kg H2 = 240 kj/mol x (1 eV/96.5 kJ/mol) x 1/(2
e) = 1.24V
This is the 1.23V (ideal) reaction voltage found in textbooks for electrolysis
and fuel cells (all reactants are assumed to be at 25C). This is energy
bond energy calculations give when all reactants are gases.
High
value
(142 Mj/kg) x (1/500) for 1 kg H2 = 284 kj/mol x (1 eV/96.5 kJ/mol) x 1/(2
e) = 1.47V
This is the 1.48V that appears in Wikipedia electrolysis article and is
approx the measured minimum voltage (1.50V at 0A) at which electrolysis
will occur. It tells us that (in effect) 0.25 volt of the 1.48 V input
energy is needed to to vaprized the water, then a bond energy calulation
(all reactants assumed to be gas) gives the remaining 1.23 V of input energy.
==============================================================================
Here's a simple way to look at the efficiency of a fuel cell. The overall reaction is simple, but there is a complication that is easy to trip over. The complication is that some of the heat released by the reaction is in a sense 'hidden', i.e. it does not show up on thermometers. This is the heat that is required to vaporize the water, its 'heat of vaprization', and it is considerable. This is an 18% term and is the difference between High and Low values of baseline hydrogen energy that references discuss. (If the hydrogen is being burned in a boiler to create heat, the energy that goes into vaporization is lost, it goes up the stack, so it lowers the efficiency of a boiler.
Seems to me water's hear of vaporization can be viewed as an unavoidable loss term in a fuel cell. The fraction of the hydrogen combustion energy used to vaporize the water output can't possible show up in the electrical output. Lets put in a kg of hydrogen into the cell and calculate the energy needed.
H2 + (1/2) O2 => H2O + (I x V) + (heat, which includes the heat of vaporization of the water)
The only (material) output of the reaction is water. The atomic weight of hydrogen is 1 and oxygen is 16, so by weight 1/9th of H2O is hydrogen. So when 1 kg of hydrogen goes into the cell, the 'burning' reaction must pull 8 kg of oxygen from the (compressed) air to yield 9 kg of water, all of which (presumably) is water vapor. (I am assuming this. Honda does mention that there is some water drips, but this could easily be condensed vapor.)
So how much energy does it take to vaporize 9 kg of water? Wikipedia (Enthalpy of Vaporization) give the heat of vaporization of water as 2.26 MJ/kg (of water)
1 kg (hydrogen) =(yields)=> 9 kg (water) x 2.26 MJ/kg (of water) = 20.3 MJ
This explains the description of the heat value of hydrgen in Wikipedia (Heat of Combustion): "The higher heating value of hydrogen is 18.2% above its lower heating value." Their energy numbers they give (below) differ by 22 MJ.
142 MJ/kg
Higher heating value
120 MJ/kg
Lower heating value
(120 x 1.18 = 142)
Bottom line
(22 Mj water
vaporizing energy for 9 kg of water/142 Mj total release energy from burning
1 kg of H2) = 15% of the energy released by the oxidation of the hydrogen
in a fuel is guaranteed to be wasted because it is needed to produce the
output water as a gas! Why is it I never read anything like this.
I think without question it is true and is a good starting point for looking
at the efficiency of the fuel cell. This 'vapor loss' term explains about
1/3rd (= 15%/42%) of the losses in the fuel cell.
Thermodynamics
--- 'burning' 1 kg of hydrogen in fuel cell
The classic
way to understand the energy released (or absorbed) by a chemical reaction
is to do a bond energy calculation. The chemical reaction in a fuel cell
is pretty simple, hydrogen 'burns' to form water [(H2 + (1/2) O2
=> H2O] and electrolysis is just same reaction running the other way, but
there are a few complications.
What are the anode and cathode reaction voltages? Fuel cell references show reaction voltage of 0V at the anode (where electrons are stripped off hydrogen) and 1.23V at the cathode (where water is created), so the (ideal) open circuit voltage of a single cell in a fuel cell is 1.23V, and this is reasonably consistent with measured data. But Wikipedia ('Electrolysis') insists the (ideal) voltage @ 0A for an electrolysis cell is 1.48V, and this is consistent with Siemens new electrolysis cell. What is going on? There were also reference to the 'high' and 'low' energy values of hydrogen, which I had never heard of.
I found a good way to look at this is just work out the total bond energy for 1 kg of hydrogen. From the formula for water (H2O) and atomic weights of O (16) and H (1), the fuel cell must be pulling 8 kg of oxygen out of the air for every 1 kg that of hydrogen that passes through the cell and into water. I find these values for the three bonds involved:
H-H
436 kj/mol
O=O
498 kj/mol (energy to break the double bond)
H-O
464 kj/mol
Here's the formula with the weight of the reactants, moles and energy. [One mol of H2 = 2 grams, O2 = 32 grams, H2O = 18 grams]
H2 (1 kg) + (1/2) O2 (8 kg) => H2O (9 kg)
500 mol 250
mol
500 mol
218 Mj 124.5
Mj
464 Mj
Energy released 121.5 Mj = [464 Mj - 218 Mj - 124.5 Mj]
Normalized per mol [Ref: 1 eV <=> 23.06 kcal/mol <=> 96.5 kJ/mol]
(121.5 Mj/500 mol) = 243 kJ/mol or 58.1 kcal/mol (57.8 kcal/mol Univ of Oxford)
So how much energy does it take to break the diatomic bonds of hydrogen and oxygen? A lot! The above numbers show almost 75% of the energy released when water is created is need to break apart H2 and O2. Chemistry references say the H2 bond is particularly hard to break because hydrogen atom, being a small atom, allows the two atoms to get close together, The O2 separation energy, figured on a per electron basis, is lower, but to separate O2 means breaking a double bond.
Explanation for 22 Mj difference
The above bond energy
calculation shows 121.5 Mj as the energy release for 'burning' 1 kg of
H2 gas, but various references say the oxidation of hydrogen yields 142
Mj. Why the 22Mj difference? Are the bond energies above wrong? Don't think
so. Univ of Oxford online chemistry site (http://www.chem.ox.ac.uk/vrchemistry/energy/page_28.htm)
does the calculation (in reverse) calculating how much energy it takes
to separate 1 mol of water into hydrogen and oxygen gas. The result they
get is 57.8 kcal, virtual identical to the 58.1 kcal, I found. And chem.ox.ac.uk
site has some information missing from the bond tables. It specifies that
H2O in the formula is a gas.
It takes a lot of energy to turn liquid water into water vapor. Wikipedia (Enthalpy of Vaporization) says:
'Heat of vaporization' of water 2.26 MJ/kg
so to vaporize 9 kg of liquid water requires 20.3 Mj = (9 kg x 2.26 MJ/kg). Here is (most of) the 'missing' 22 Mj. The difference in energy between liquid water and water vapor is a classic complication when a burning reaction yields water. It's why there are two heat values for hydrogen: 'high' and 'low'. If you are burning hydrogen in a boiler for heat and the water vapor goes out the stack (without condensing), you lose about 15% of hydrogen's (potential) energy value, because the water vapor takes this heat out the stack. In this application the heat the boiler puts out is the 'low' energy value of hydrogen.
Electrolysis
Electrolysis
is just the fuel cell reaction run backwards. We can use the results of
our calculations above to figure the theoretical voltage(s) for electrolysis
(of water). Another unit for energy is eV where [1 eV <=> 23.06 kcal/mol].
If one electron was required per water molecule, then this relationship
gives the actual (measurable) voltage, but it takes two electrons per water
molecule to make the reaction (either fuel cell or electrolysis) go, so
with double charge we divide the voltage by two.
My bond energy calulations for water as a gas gave 58.1 kcal/mol
voltage (H2O gas) = 58.1 kcal/mo x 1 eV/(23.06 kcal/mol) x
(1/2e) (two electron per H2O)
= 1.26V (pretty close to 1.23V textbooks)
To get the voltage for liquid water we need to scale up by 18% or so to account for the extra energy required to vaporize the water.
voltage (H2O liquid) = 1.26V (gas) x [(120.5 Mj + 22 Mj)/120.5 Mj]
= 1.26V (gas) x 1.183
= 1.49V (very close to the Wikipedia 1.48V)
In other words thermodynamically electrolysis of water looks to be equivalent to two step process: a) vaporize the water, b) rip the gaseous H2O apart into its two gases. The energy for the first step is about 18% (22 Mj/120.5 Mj = .183) of the step b). The bond energy calculation for the gas ripping comes out close to 1.23V (I get 1.26V), and when this is scaled up by 1.18 to account for the extra energy to be input to vaporize the water out comes 1.49V, consistent with Wikipedia.
a) H2O (l) => H2O(g)
0.23V
18% of step b)
b) H2O(g) => H2 + (1/2) O2
1.26V
(1.23V ideal)
-----------------------------
total 1.49V
(1.48V ideal)
==============================================================================
So how much CO2 emission is associated with the making of hydrogen? When hydrogen is made by electrolysis of electricity, the 'waste' product of the electrolysis is oxygen, which can either be sold or if no market released into the atmosphere. Clearly CO2 emissions depend primarily on the source of the electricity, plus some additional CO2 associated with transport and compression (or liquification) of the hydrogen. This is the same as an electric car, except there will x3 times more emission, because the efficiency of the hydrogen cycle is so poor that x3 times more electricity per mile is needed for a hydrogen car compared to a battery powered electric car.
Natural gas reforming
However, as
every reference points out, at present most hydrogen (96%) is made not
by electrolysis but is manufactured from natural gas. To avoid transport
nearly all of it is made adjacent to chemical plants that will turn it
into something else like fertilizer (via amonia). The industrial chemists
desecribe it as two reactions (that run at different temperatures), but
clearly they can be combined (eliminating the CO) to give a simple formula
where the carbon and oxygen both separate from their hydrogen (disassociate
in presence of a catalyist) and join together form a molecule of CO2 with
the hydrogen atoms joining up into H2 gas. Anther way to put it is, with
energy input oxygen can made to jump from the more favorable hydrogen (H2O)
to the less favorable carbon (CO2).
Industrial chemists say the reforming is the following two reactions. The first reaction (separating H from both C and O releasing 3H2 with the C combining with the O to form carbon monoxide) is strongly endothermic, meaning a lot of energy input (high temperature) is needed to drive the reaction, but that much of the energy is recovered in a second reaction (as CO is able to pull water apart converting CO to CO2 and releasing the hydrogen from the water as a molecule of H2). I worked out the bond energies and that is correct.
CH4 + H2O =>
CO + 3 H2
612 => 496 116 kcal (endothermic)
CO + H2O
=> CO2 + H2
407 => 477 70 kcal (exothermic)
--------------------------------------------------
CH4 + 2 H2O => CO2
+ 4 H2
832 => 786 46 kcal (energy
input needed to drive reaction)
4 x 98 + 4 x 110 => 2 x 187 +
4 x 103
Equivalent set of reforming equations
I played around
with the equations and see that reforming equation can also be obtained
another way that gives some insight as to what is going on. The first equation
below is simply the burning of methane to yield CO2 and H2O. The second
equation is the electrolysis equation where H2O is separated into O2 and
2H2. Doubling the electrolysis numbers gets the O2 to cancel yielding the
reforming equation where methane gas combines with water to make CO2 and
H2. (Check: net energy of to drive the reaction in both sets of
equations comes out to be 46 kcal.)
Another interpretation, which I imagine would make a chemist cringe, is that reforming works this way: With energy input water is torn apart into oxygen and hydrogen, just like in electrolysis, and the oxygen released is exactly the amount needed to burn the methane to CO2 and water. The water output from the methane burning simply serves to reduce the amount of water (steam) that must be supplied externally.CH4 + 2 O2 => CO2 + 2 H2O 624 => 814 190 kcal (energy release)
4 H2O => 2 O2 +
4 H2
880 => 644 236 kcal (energy
input)
8 x 110 => 4 x 103 + 2 x 116
-------------------------------------------------------------
------------------------------
CH4 + 2 H2O => CO2
+ 4 H2
46 kcal (energy input)
The nice thing about this set of equations is we can see where the energy contained in H2 manufactured is really coming from. Here hydrolysis (at least conceptually) is making all the hydrogen, and to made four moles of hydrogen it needs an energy input of 236 kcal. Methane when burned puts out a lot of energy, 190 kcal of the needed 236 kcal. Thus it seems clear that in reforming what is going on is that about 80% (190 kcal/236 kcal) of the energy contained in the manufactured hydrogen gas is coming from the methane, which is (in effect) being burned, with the remaining 20% (46 kcal/236 kcal) beging supplied (as heat) to make the reaction go. (Of course all these numbers assume 100% efficiency, which never happens.)
1 kg H2 energy check
Are these
bond energy numbers consistent with the energy contained within 1 kg of
H2. Ans: yes. The result comes out to be very close to 120 Mj, which is
the low heat energy value of hydrogen. This means that the H2O in equations
is assumed to be a gas, i.e. steam. {I still have some confusion over how
'/mol; here is to be interpreted, when 1 mole of methane goes in and four
moles of hydrogen come out, but the numbers come out right if it assumed
that 46 kcal of energy yield 8 grams of hydrogen (4 H2).}
236 kcal x (4.184 kJ/1 kcal) x (1,000 gm/8 gm) = 123 Mj (120 Mj is low heat value of 1 Kg of hydrogen)
CO2 perspective
The reforming
equation (above) shows that (ignoring the source of the 46 kcal of energy)
the manufacture of 1 kg of H2, which is 500 moles, creates 125 moles of
CO2. 1 mole of CO2 is 44 grams = [2 x 16 + 12]. References say 1 gal of
pure gasoline (no ethanol) or octane C8H18 when burned releases 19.64
lb of CO2. This is about x3.1 times the weight of the gasoline since each
carbon (84% weight of octane) picks up two oxygen in the burning. Converting
the 19.64 lbs of CO2 to moles we get 203 mole = [(19.64 lb/2.2 lb per kg)/0.044
kg/mol], so summarizing:
1 kg H2 created by reforming methane
=> 125 moles of CO2
1 gal of C8H18 (gasoline) burned
=> 203 mole
of CO2
The (oxidation) energy of one gal of (pure) gasoline is almost exactly the same as a kg of H2, but because the efficiency of a combusion engine is 2-3 times lower than a fuel cell, the driving range of a hydrogen car on one kg of H2 is x2-3 higher than one gal of gasoline. Put another way in terms of range 1 kg H2 <=> 2-3 gal of gasoline. We get the same result another way. The current generation of hydrogen cars go about 60 miles on 1 kg of H2 (240 mile range with 4 kg tanks), but the driving range of a typical car on a gal of gas is 20-30 miles.
Thus in terms of equivalent range we should compare the CO2 released in reforming methane to make 1 kg of H2 with the CO2 released by the burning of 2-3 gal of gasoline. This is a ratio of
4.1 = (203 mole of CO2/125 mole of CO2) x 2.5 gal (av) per kg of H2 CO2
Much less CO2
Bottom line running
a hydrogen car on hydrogen manufactured by reforming methane does release
CO2 (from the reforming), but it is only about 25% as much CO2 as a typical
car would release to drive the same distance. The hydrogen car CO2 release
is low partly because the source of the energy for hydrogen is natural
gas, which has the lowest carbon content per joule, and partly because
combusion engines are so inefficient such that 2-3 gal of gasoline must
be burned to drive the same range as 1 kg of H2.
Energy of gasoline
References
say the energy value (low heat value) of a (US) gal of gasoline is 120
Mj, which coincidentally happens to be the same for a kg of H2. The equation
is for burning of pure gasoline or octane is below. Let's check the energy
released with octane is 100% burned. A quick look at the molecule on Wikipedia
'octane' show it is a chain of 8 carbon atoms festuned with hydrogen that
tie the carbon loose valence bonds (C-H). All the carbon in the chain are
single bonded to each other (C-C). 18 H result from the 6 interior C each
having 2 H and the two end C each having 3 H.
2 C8H18
+ 25 O2 =>
16 CO2 + 18
H2O 7,548
=> 9,944 2,396 kcal (energy release)
2 x
(7 x 80 + 18 x 98) + 25 x 116 =>
16 x (2 x 187) + 18 x 2 x 110
Two moles of octane weighs 228 gr = [2 x 12 x 8 +18] and a gal of gasoline weights 2.76 kg (approx), which is a ratio of 12.1, so the energy release from burning a gal of (pure) gasoline is
12.1 x 2,396 kcal x 4.184 kJ/kcal = 121 Mj check (ref give 120 Mj as LHV of octane)
Basic chemistry for hydrogen source
Their chemical
formulas give a clue as to why the starting point for making hydrogen is
either methane (CH4) or methanol (CH4O). Methane is a gas whose simple
molecule is four hydrogen atoms bonded to a center carbon atom each to
one of its four valence bonds. Methanol adds an oxygen turning it into
a liquid at room temperature. From basic chemistry (and the look of its
equation) its molecule is essentially methane except one of the H bonded
to the carbon is replace by an OH, one oxygen valence bond to the carbon
and the other to the hydrogen. In other words its methane with an oxygen
wedged between the carbon and one of the outside hydrogen.
methane (gas)
CH4
menthanol (liquid)
CH4O (or CH3OH)
==============================================================================
So I'm glancing through WSJ and an article catches my eye that a large fraction of new houses built in Japan now an alternate source of electricity built in, either solar panels or a fuel cell. The article says 40,000 homes have had fuel cells installed! What, a production PEM fuel cell? They are pushed by Tokyo Gas, which even runs TV comercials for them. My first thought is where do they get the hydrogen.
Panasonic Ene-Farm Residential Fuel Cell
Turns out the Japanese
unit is the 'Ene-Farm Residential Fuel Cell' made by Panasonic (fuel cell
only). It was introduced a few years ago with a new model in 2013. It puts
out 200 to 750 watts of electricity and uses the waste heat to make hot
water. Panasonic claims the efficiency is 95%, and it is this high because
a) Panasonic specificies 95% efficiency relative to 'Low heat value'
b) Co-generation unit, waste heat from the fuel cell is captured used to
heat water
Cost? Super expensive. It costs 15k to 20k says the article of which 1/3rd (or more) is subsidized. Panasonic prices are in the low 20ks. The claim in the article is that by 2016, Panasonic hopes to be able to cut the price by more than half to 7k.
Hydrogen?
So where do the
houses get the hydrogen? Aparently from the city gas! The spec implies
that Toyko city gas is a mixture of hydrogen and methane and this unit
is somehow able to separate out the hydrgen for the fuel cell. Interesting...
Description
Look at the
size of this thing! It's much larger than a hydrogen car fuel cell, yet
it only runs at about 1% of the power level (750 watts vs 80 kw).

Panasonic Ene-Farm Residential Fuel Cell (2013 model)
(far left, hydrogen separator and fuel cell
(right two, hot water tank and backup heat source)
40,000 have been installed in new Japanese homes
High temperature fuel cell for backup electrical power
from gas
A new US company
(Redox) has put out specs for an 80 kw (1 meter cube, 750 lb) fuel cell
that operates off gas and generates 80 kw. (Of course, it doesn't exist
yet, but at least they have a spec and a model.)
Proposed Redox 80 kw gas powered fuel cell
32 modules x 2.5 kw/module (11V x 227A)
(550C operating temp!)
Solid oxide fuel cell
This thing
can't be a PEM fuel cell as its operating temperature is super hot 550C.
Yup, a little digging shows this is a 'solid oxide' fuel cell, which has
apparently been around for a long time. This type of fuel cell runs HOT
(800C) and can run on natural gas as well as hydrogen. Redox twist is some
new engineering tricks to 'lower' the temperature to 550C, which reduces
the cost of materials, and to lower the amount of rare earths needed.
Solid oxide fuel cell technologyRedox white paper: http://www.redoxpowersystems.com/wp-content/uploads/2012/07/Science-2011-Wachsman-935-9.pdf
A solid oxide fuel cell works backwards from a PEM fuel cell in this sense: In a PEM fuel cell it is the fuel (hydrogen) that is ionized, the H+ hydrogen ion after 'drifting' across the membrane meets O2 on the other side to form water. Since a PEM membrane can only conduct protons, the fuel is limited to hydrogen. But in solid oxide fuel cells it is the oxygen gas that is ionized. O2 is reduced at the cathode (breaks apart and accepts external electrons becoming O2- ions), and the O2- ions 'drift' (most likely) across the rare earth solid oxide membrane. When the O2- ions emerge, they are able to oxidize almost any gas fuel (methane, hydrogen, etc). If hydrogen is used, then only water comes out, if a hydrocarbon gas fuel is used, then water and CO2 come out.The white paper below give the efficiency as only 45% to 65%, and that is based on the lower heating value the fuel, no better than, and probably worse than, PEM fuel cells. The paper points out that the theoretical efficiency of fuel cells, in contrast with the carnot cycle, increases as operating temperature decreases, which means the low operating temperature of the PEM fuel cell is an advantage.
=================================================================================
Draft summary
I saw a repeat
2011 Nova show and it showed a woman who drives a GM hydrogen car refueling
with hydrogen. She said it refuels in a few minutes just like a regular
car and will go 200 miles. Leno has one too, said he had been driving it
for two years, nice car. Well that go my attention, hydrogen cars actually
exist, they are on the road. GM built 100 hydrogen cars in 2008-9 and Mercedes,
pushing hydrogen hard, has recently made about 600 that it leases in Germany
and in LA. So high power fuel cells that are reasonably reliable exist,
on board hydrogen storage for reasonable range exists, and hydrogen refueling
stations that can refuel cars fast exist. So when are we going to see production
hydrogen cars? What are the problems? I set out to find out.
--------------------------
There are five major players: GM, Mercedes, Toyota, Honda, and Hyundai. Until recently all had been promising a production hydrogen car in 2015, but GM and Honda have just bailed, announcing they are going to start work together (lowering R&D costs) and saying maybe a production hydrogen car by 2020. However, Mercedes, Toyota and Hyundai are still expected to introduce a production hydrogen car 2014-15. Detail specs of four of their prototypes (all except Toyota) are available. Turns out they all are making basically the same car, a four door sedan or small crossover on a 110” wheelbase with 100 kw of power.
Inside they are basically the same too. Their fuel cells may be different, but they all store hydrogen in the same tanks and refuel the same way. Regulatory standards for tanks and refueling have been fully developed in the last ten years. Car companies do not have to engineer how to store hydrogen, they just buy a class IV tank high pressure hydrogen tank (10,000 psi) of the size they want, knowing it will meet detailed safety standards.
A hydrogen car is usually described this way --- It's like an electric car except the the battery is replaced by a fuel cell powered by an onboard hydrogen supply. It is very efficient, x3 more efficient than an internal combustion engine, and no pollution since hydrogen and oxygen combine to make only water.
Is this true? Yes, but it is also misleading. For one thing what replaces the battery is not just the fuel cell, which is kind of flow through battery, but a fuel cell in parallel with a hybrid size (1-2 kwh) lithium ion battery. Fuel cells don't run backwards (you cannot pump in electricity and get out hydrogen!), so to capture braking energy a battery is needed. The battery also outputs substantial peak current during car acceleration, thus lowering the peak power (and cost) of the fuel cell. It is used to power accessories and can run the car at low speed, allowing the fuel cell to be shut off when the load is low.
1) Hydrogen cars are expensive to manufacture because both the fuel cells and hydrogen storage tanks are expensive. A fuel cell is actually a stack up of 440 cells in series, since each cell's output voltage is only about 0.7V. Each cell has to be provided with a flow of hydrogen gas and (compressed) air. The gas comes in via a terminal, called a bypass plate, with channels cut into it on one side for hydrogen and the other side for air. It must be strong, thin, have very low resistance (since it carries the full 300A) and be impervious to gas diffusion. Nobody yet knows how to manufacture this thing economically in volume and each fuel cell has 440 of them. The heart of each fuel cell is a triple thin film sandwich which blocks hydrogen, but will allow the H+ ion to diffuse through. To improve the efficiency and improve the flow rates both the anode and cathode of the sandwich use platinum as a catalyst, about 1k+ worth. People have looked for other catalysts for years, but platinum works best, there is no replacement for it on the horizon. Hence the heart of the fuel cell where the electro-chemistry happens and the voltage is generated is expensive. Finally 60% efficiency means the fuel cell is dissipating a lot of heat so in addition to the two gas channels in/out to each cell there must be a liquid coolant channel too. The whole thing is a complex honeycomb using materials that are either expensive or hard to manufacture.
The hydrogen storage tank(s), which mount under the floor, are expensive because they operate and must be safe at huge pressures (10,000 psi, x680 atmospheric pressure). Hydrogen embrittles metal so modern high pressure (class IV) tanks are now made entirely from carbon fiber. There are hints that a tank big enough to hold 4 kg of hydrogen (enough for 240 miles range) might cost currently 5-10k.
2) The hydrogen guys efficiency claim is true, but misleading. Gasoline engines are horribly inefficient outputting as mechanical work only about 20% of the potential energy in gasoline, whereas a fuel cell will extract 60% of the energy in the hydrogen, hence the claim that it is x3 more efficient than a gasoline powered car is true. However if the efficiency of hydrogen electric car is compared with a battery powered electric car the situation looks very different. There are a lot of losses associated with making hydrogen then using the hydrogen to make electricity (for the electric motor). These are both electro-chemical reactions involved, neither of them particularly efficient (75% and 60%) and more energy is lost compressing the hydrogen to fit into the car tank(s). The result is that a hydrogen fuel cell electric car uses about x3 times more electrical energy per mile than a battery powered electric car.
3) You can't recharge a hydrogen car at home (horribly expensive), you must live near a hydrogen refueling station. This makes for a nasty chicken and egg problem to get hydrogen cars introduced.
4) Prototype
hydrogen cars have demonstrated two (potential) advantages battery powered
electric cars: range and refill times, both comparable to a normal car.
But for some reason (maybe cost) the hydrogen cars being brought to market
have only half the range they could have if they had a 2nd tank. This throws
away one of their two advantages since their range of 240 miles is no better
than the Tesla Model S sedan.
---------------------------------------------------------------------------------------------------------------------------------------------------------