How to Estimate the # of Cells that make up a Human
Body?
Trick --- 'C. Elegens worm' is a 1 mm long
nematode that has been studied extensively at MIT. It's cellular development
from a single cell to complete animal has been mapped out. What has made
this possible is two things. First, it is so small it has only
about 1,000 cells. Second, its cell growth pattern is always exactly
the same, and being a 'real' animal it has a variety of cell types: skin,
nerve, and gut.
Scaling ---1) Human body has roughly the same form
factor (height/length to width ratio) as this little worm. We'll also assume
that on average worm cells and human cells are the same size
2) Ratio of human height (2 m) to worm length (1 mm) is about 2,000
Scaling in three dimensions
Human cells
= 2,000 x 2,000 x 2,000 x (1,000 cells of worm)
= 8 x 10^ 9 x (10^3 cells of worm)
= 8 x 10^12
approx
= 10^13
So scaling from C. Elegens worm the human body is made up of (10 to 13th power) cells or 10 trillion cells.
How good is our estimate?
For such a
simple procedure and simplifying assumptions, pretty good. Web search show
no one knows how many cells (not including bacteria) are in the human body,
but estimates run from 10 trillion to 100 trillion.
10 trillion -- American Biotech. Laboratory,
& Bulls Press, & How Stuff Works
100 trillion -- Med Info Source, & Howard
Hughes Medical Institute, & NASA Origins
How Many Cells are in the Human Body Really (including
'Bugs')
Relatively
recent work indicates that over 90% of cells within the human body are
foreign. That's right, most of the cells in our body are not human,
do not have human DNA, they are germs, more specifically the fauna and
flora of the gut and a few other regions. All our germs don't weigh that
much because germ cells are much smaller than human cells, but nevertheless
it is estimated that 30%-40% of the human gut by weight is germs.
Scientific American (1/09) says that metagenomic analysis of the human intestinal tract finds x100 more genes than exist in the human genome.
Germs are Everywhere in the Human Body, Right?
Actually,
no. While much of the gut is germs and there are lots of germs in
the mouth and on the skin, the inside of the abdominal cavity and inside
of limbs is germ free, sterile. That's why a burst appendix is so dangerous,
it puts germs where there shouldn't be any.
Why do Shit Turds look like that?
Isn't it a
minor mystery why shit turds look (about) the same regardless of what we
eat? (Beets are one exception) The foreign fauna and flora cells
in our gut are continually growing and dying. A huge number of them are
expelled when we shit. A substantial fraction of shit turds is composed
of these 'dead germs'. Maybe this explains why shit generally looks about
the same regardless of what we eat!

Prokaryotic Cells vs Eukaryotic Cells
Human 'bugs'
(human fauna and flora) are single cell creatures. Those classified as
bacteria & archaea (if there are any archaea in humans) have the less
structured prokaryotic cell type. All the other single cell bugs, plants
(including algae), animals, and fungi, have the more structured eukaryotic
cell type with DNA segregated in a membrane enclosed nucleus.
There are lots of other differences between cell types. The first three links below give a good overview comparing cell types.
http://www.bookrags.com/sciences/genetics/cell-eukaryotic-gen-01.html
http://www.bookrags.com/sciences/genetics/eubacteria-gen-02.html
http://www.bookrags.com/sciences/genetics/archaea-gen-01.html
http://library.thinkquest.org/C004535/different_cell_types.html
x10 smaller
Eukaryotic
cells have wide range of sizes, but the average is about 25 microns in
linear dimension, which a little math will show is consistent with our
10 to the 13 power for the number of cells in the human body. Bacterial
prokaryotic cells are much smaller, typically about 10 times smaller
in linear dimension or 2.5 micron.across.
This cell size difference explains why the human body can have so many foreign cells inside. You can pack 1,000 bacteria into the volume of one typical human cell!
Cell trivia from Wikipedia --- human body has about 210 types of cells.
Yeast
Highly studied
representative organisms of their type are called model organisms. A model
prokaryotic organism is the gut bacterium ecoli (escherichia coli)
Surprisingly, a model organism with (the much more complex) eukaryotic
cell type is bakers yeast (saccharomyces cerevisiae). Bakers yeast
has 6,000 genes and shares about 23% of its genes with humans! (Humans
only have about 30,000 genes).
Is the yeast in a package alive? The answer given is always yes, but how can yeast live in a dry package for a year or more? The only explanation I can find is this: "Active dry yeast is yeast cells clumped into tiny, dehydrated granules. The yeast cells are alive, but dormant because of the lack of moisture." So not only can yeast be dehydrated and still live (or come back to life), but (according to cook books) it can be frozen too and still live (or come back to life)! Yeast can also form spore which can survive (if dry) forever. When spores fall into a nutrient solution, they germinate into yeast cells.
A baking test to see whether yeast will make dough rise is called 'proofing' the yeast. Yeast and sugar are mixed in warm water. The yeast ferments the sugar releasing CO2 and alcohol. If the yeast is alive, within 5 min visible bubbles will be seen! When dough is left to rise, the carbon dioxide from the yeast is trapped within tiny bubbles and results in the dough rising.
Fermentation is a type of anaerobic (without oxygen) respiration. Fermentation is thought to have been the primary means of energy production in earlier organisms before oxygen was at high concentration in the atmosphere ,and thus would represent a more ancient form of energy production in cells. Fermentation products contain chemical energy (they are not fully oxidized) but are considered waste products since they cannot be metabolized further without the use of oxygen. (Fermentation produces 2 ATP molecules per molecule of glucose compared to approximately 36 ATP by aerobic respiration.)
Fermentation
C6H12O6 --> 2C2H6O
+ 2CO2
+ (energy in 2 ATP)
glucose (sugar)
--> ethanol (alcohol) + carbon dioxide + energy (ATP)
(Energy Released:118 kJ/mol)
Archaea
Another interesting
cell tidbit is that if a cell produces methane it means it must be in the
archaea family. Archaea are the recently recognized 3rd domain of life
and probably the oldest in existence. A lot of methane, a greenhouse gas,
is known to come from cow's belching (50 liters a day per cow!), so there
must be archaea in cows. That means it's probably a good bet we have some
archaea living inside us too.
Farts
Farts can
be ignited because they contain flammable gases, but what are these gases?
Well, reportedly the gases are hydrogen and in 1/3 of people methane. Methane
reportedly burns blue. Most fart reference say that the methane comes from
gut bacteria, but this cannot be true because bacteria don't make methane.
Only archaea make methane. Conclusion: 1/3 of people must have archaea
in their guts.
Everything you ever wanted to know about farts is here: http://www.heptune.com/farts.html
Gut microflora
Studies
of the human gut microflora are just beginning because only recently have
the techniques to analyze this huge complex mess of 'foreign' DNA become
available. So without doubt some of the stuff being now written about gut
microbes and all the wonderful functions they perform for us is to some
degree speculative.
(NY Times 8/12/06) --- Of the trillions and trillions of cells in a typical human body — at least 10 times as many cells in a single individual as there are stars in the Milky Way — only about 1 in 10 is human. The other 90 percent are microbial. These microbes — a term that encompasses all forms of microscopic organisms, including bacteria, fungi, protozoa and a form of life called archaea — exist in the ears, nose, mouth, vagina, anus, as well as every inch of skin, especially the armpits, the groin and between the toes. The vast majority are in the gut, which harbors 10 trillion to 100 trillion of them.
Known collectively as the gut microflora They help create the capillaries that line and nourish the intestines; produce vitamins, in particular thiamine, pyroxidine and vitamin K; provide the enzymes necessary to metabolize cholesterol and bile acid; and digest complex plant polysaccharides, the fiber found in grains, fruits and vegetables that would otherwise be indigestible.
In the womb, humans are free of microbes. Colonization begins during the journey down the birth canal, which is riddled with bacteria, some of which make their way onto the newborn’s skin. By about the age of 2, most of a person’s microbial community is established, and it looks much like any other person’s microbial community.
Exploding atoms in the body
My
essay on atoms shows that about 8,000 radioactive atoms (mostly potassium
40 and carbon 14) explode in your body every second. And interestingly
most of these atomic 'explosions' in your body emit anti-matter.
Potassium 40 (@ 4,400 decays/sec) actually decays in three ways. It decays down the periodic chart (to element 18 argon 40) in two ways: by spitting out a positive charge or by sucking in a negative charge, both are a type of beta decay that via the weak force converts a proton into a neutron, and potassium 40 decays up the periodic chart (to element 20 calcium 40) by emitting an electron. The positive charge emitted is an anti-electron (positron). Whether it makes it out of the atom or whether it hits an electron on the way out is a good question, but since isotopes that emit positrons are routinely used in medicine (positron emission tomography), there is a good chance the positron does make it out through the atom's electron cloud. The long half life of potassium 40 (1.25 billion years) has led to potassium 40 to argon 40 decay being widely used as a dating tool for very old rocks.
The negative charge sucked into the nucleus during the decay of some potassium 40 is actually one of the atoms own inner electrons (K or L orbit). That's right the atom 'steals' one of its own electrons to convert a proton into a neutron! While this leaves the argon atom with the right number of electrons, they are in the wrong orbits so the atom is excited and must emit energy.
The decay of potassium 40 to calcium 40 is classic beta decay (now called beta minus decay) where via the weak force a neutron converts into a proton (via an intermediate W boson as a down quark converts to an up quark) and emits an electron. This type of decay also emits an anti-neutrino. Carbon 14 in the body also decays this way as does rubidium 87 the third most common body radioactive atom, and curiously all beta minus decays emit anti-neutrinos with a right hand spin. Thus the vast majority of atomic decay in the body emit anti-neutrinos, hence the human body 'radiates' about 8,000 anti-neutrinos into the world per second!
Heavy exploding atoms in the body, like lead, radium and uranium, tend to decay via alpha emission. There are only a couple of dozen alpha explosions in the body per second. A high energy alpha particle fired from an exploding atom in dense material can go about 25 microns (25 x 10^-6 m or 1/40 mm), which is the diameter of a typical cell. So this type of exploding atom probably destroys one or two cells.
8,000 exploding atoms per second sounds bad, but it needs to put into perspective by considering that many more cells than this die per second from 'natural' causes. Here is a detail reference on cell count and cell death rate. Its estimate for cell death per second is between 200,000 to 3 million (!), the lower number for body structure with the higher number including all the fluid in the body.
http://www.madsci.org/posts/archives/2001-02/981770369.An.r.html
Human body radiation exposure (March 15, 2011)
The partial
meltdowns and radiation leakage from several Japanese reactors following
a huge 9.0 earthquake and tsunami have revived my interest in radiation
levels. Radiation units are confusing because there are so many of them
and new ones seem to pop up. It looks like (currently) the right unit to
use for human body radiation dose is the sievert (Sv).
It's the right unit for health because it (attempts to) scales raw physical
radiation levels to reflect biological effects. Single event does are typically
microsievert (uSv) and millesievert (mSv).
Yikes, as I suspected doses levels are a zoo. Up comes an EPA document "Radiation risks and realities" and all its does are in millerem! The conversion is [1 uSv = 100 urem], in other words sievert levels are 100 times smaller than rem levels. (In the Tech a nuclear engineer writing about Japan also uses rem.)Background dose and average radiation dose
Safe levels
EPA (Wikipedia 'Sievert'
has same numbers) gives annual radiation does at sea level (cosmic
+ terrestrial) as 50 mrem (500 uSv), but radon in homes and body radiation
need to be included and says average background radiation is x4 higher
(200 mrem, 2mSv), which is a daily background radiation dose of
6 uSv.
Background (only)
Daily background dose
6 uSv (0.6 mrem)
Annual background dose
2 mSv (200 mrem)
-- In Tokyo, a metropolis of 13 million people, a Reuters reading on Saturday morning showed ambient radiation of 0.22 microsieverts per hour (5 uSv/day), about six times normal for the city. However, this was well within the global average of naturally occurring background radiation of 0.17-0.39 microsieverts per hour (4-9 uSv/day), a range given by the World Nuclear Association. (NYT 3/26/11)Average radiation dose (round #'s) (1/2 background & 1/2 medical)
Average radiation dose
In a NYT article
by David Sanger about high radiation levels from Japanese nuclear plants
the reference for the average radiation dose of USA residents (background
and medical radiation combined) is the Nuclear Regulatory Commission: 0.62
rem. This is x3 higher than the above background dose. I searched out the
Nuclear
Regulatory Commission site. Why so high? Because half (0.31 rem) is
background and half (0.31 rem) is medical radiation. Their background is
50% higher than above because they have high radiation from radon (which
is very variable!).
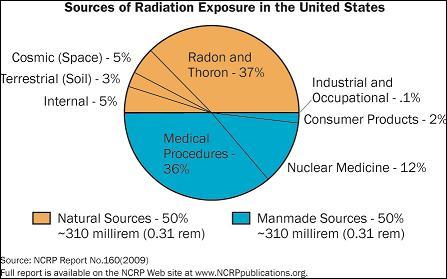
Note half the average radiation dose (above) is background
(orange).
It 50% higher then EPA value, probably because radon
levels are so high.
(source -- Nuclear Regulatory Commission)
ThoronAirport full body scanners --- one banana dose
What the hell is 'thoron' (see above graphic)? Some new element (!) or did the NRC write thoron when it meant thorium? Turns out 'thoron' is really the element radon, an informal (stupid) name for the 220 isotope of radon, part of the thorium decay chain with a 1 min lifetime.The thorium 232 to (stable) lead 208 decay chain is interesting. Thorium 232 goes to radium 228 with a 6 year lifetime followed by 8 more decay produces all with faster lifetimes (two years to seconds). One of its decay products is radon 220 (so-called gas thoron). Thus thorium decays will typically be a mixture of all nine intermediate unstable products outputting a total of five alphas and four betas.
banana
0.1 uSv
airport full body (backscatter) scanner
0.1 uSv
Why banana? Banana's have a lot of potassium (450 mg) and 0.01% of naturally occurring potassium (K40) is radioactive (1.3 billion year half life). And potassium is the most radioactive element in the human body.
So the airport full body scan radiation dose is 1/60th the daily background does, which says the scanner radiation level is nothing, a banana equivalent. It is equivalent to 2 minutes of radiation during flight!
Japanese reactor meltdowns (3/15/11)
The press is reported
today (its appears in Wikipedia 'Sievert' too) that radiation levels outside
one of the Japanese plants have been reported as high as 400 mSv/hr (40
rem/hr). So how high/dangerous is this? As usual the press reports
give no clue. (Tech engineer says the highest recorded dose by any worker
on the site is 11 mrem. Seems awfully low, this is just x11 daily av dose.)
Main radiation emitter from damaged nuclear power plant
iodine 31
8 day half life (essentially gone in 3 months) (beta, gamma, 1 Mev)
(accumulates in the throid gland, especially in growing children)
cesium 137
30 year half life (beta, 1.2 Mev)
-- Heavy contamination with cesium 137 is in large part what forced Soviet authorities to create a nearly 20-mile, uninhabitable exclusion zone around the Chernobyl reactor site.) Wikipedia shows that after a year or so the only significant radiation source from Chernobyl was cesium 137 (plus some cesium 134 and a tiny bit of rubidium). Chernobyl was a steam exposion with the reactor going full blast. In comparison the three operating Japanese reactors all shut down immediately at the earthquake.
Cesium is alkali metal (col 1) element, like potassium and sodium, and like potassium if in the environment it goes into fruit and vegatables. It is expelled from body in about three months (sweat and urine).
Effects to humans of acute radiation (within one day)
Numbers
from Wikipedia 'sievert'. It doesn't say, but I presume these doses are
assumed to be absorbed by the whole body.
0–0.25 Sv: (25 rem) None (0.1 Sv
or 10 rem says NRC)
In other words a safe radiation dose is 25,000 daily dose (25 rem/1 mrem)
National Academy of Sciences says 0.1 Sv (10 rem) single does give 2% cancer
risk increase
--Three contract (what?) workers suffer radiation exposure of roughly 170 millisieverts (0.17 Sv) after radioactive water gets into their boots while they are laying a new cable. Two are taken to a hospital with radiation burns. The three workers exposed to high levels of radiation, who are fully conscious and ambulatory, are transferred to the National Institute of Radiological Sciences for monitoring. (NYT MARCH 24, 2011)
0.25–1 Sv: Some people feel nausea and loss of appetite; bone marrow, lymph nodes, spleen damaged.
Since the exposure was local to the feet and legs, I don't know what the reported 170 millisieverts means. Is this a local dose, in which case it would be much higher locally than this table, which I presume is spread evenly over the human body? Full body vs local exposure makes dose numbers difficult to interpret.
Chernobyl dataJapanese reactor (New Yorker Oct 2011)
134 plant workers and firefighters battling the fire at the Chernobyl power plant received high radiation doses – (800 mSv to 16 Sv) – and suffered from acute radiation sickness. Of these, 28 died within the first three months from their radiation injuries.
100 millesievert
5 yr does limit of workers at plant prior to meltdown
250 millesievert
raised limit after meltdown, when it was clear 50 millesievert for many
workers would be exceeded, "any higher would be unthinkable".
5 sievert
Leaking pipe at reactor remains a hot spot. 10 sieverts/hr or a leath dose
in 30 min
Hard data (3/19/11)
Here finally is
some real and realistic radiation data. Levels need translating. Values
at the plant gates are given as (round numbers) 300 uS/hr (30 mrem/hr).
This is 700 mrem/day, which is x700 normal total daily radiation. About
a month at this level is the threshold for radiation damage (single dose).
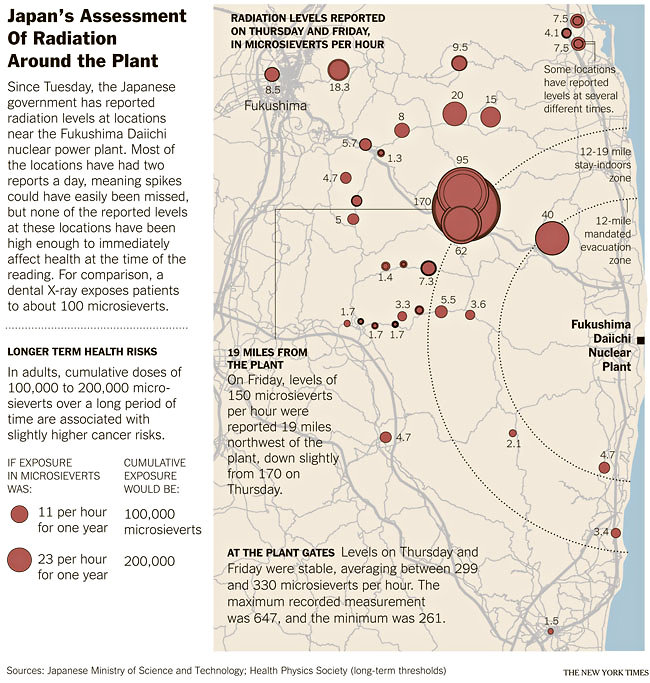
Hot spot (big red circles) 19 miles from plant is
about half the level at the plant gate! (3/19/11)
I assume this is the radioative steam and releases
blown inland.
Most of the small circles are 7 to 15 times the normal
radiation rate,
and not a real problem.
(Left) Cancer threshold of 100,000 to 200,000 uSv
= 10 to 20 rem
(10 to 20 rem is roughly the threshold for radiation
damage for a single dose)
Denver (sustained) background level
A good reference
for evaluation of radiation levels from a reactor accident is (what I call)
the 'Denver background' level. Living in Denver gives a sustained (lifetime)
higher level of radiation and the NRC says, " People living in areas having
high levels of background radiation – above 1 rem (10 mSv) per year – such
as Denver, Colorado, have shown no adverse biological effects."
What, 1 rem! Princeton says, "The annual dose from cosmic radiation in Denver is 50 millirem while the annual dose at sea level is 26 millirem." In other words living in Denver adds annual dose only 25 mrem (250 uSv). For reference annual background dose is 200 to 300 mrem. This doesn't contradict NRC reference (above), but the implication is that Denver's level is x40 higher than it actually is. Or put another way you can increase background levels x20 higher than Denver without known damage.
Single dose threshold
NRC
says, "there are no data to establish unequivocally the occurrence of cancer
following exposure to low doses – below about 10 mrem (100 mSv).
The EPA level for no injury (does this include cancer after a long delay)
is x2.5 higher 25 rem (250 mSv).
A letter to NYT (May 2011) quotes a National Academy of Sciences report (below) indicating that the threshold of injury from a single does is about 10 rem, the dose which at 1 mrem/day would take 30-40 years. It causes a 2% increase in cancer risk.
-- Our National Academy of Sciences has a standing committee on the effects of radiation. Its most recent report, “Biological Effects of Ionizing Radiation VII,” concluded that a single dose of 100 millisieverts (10 rem), 40 times the average annual exposure to natural background radiation, increases a person’s chances of cancer by about 2 percent.C. Elegens Worm
The C. elegans body and eggshell are transparent so that each cell can be visualized. Another advantage of C. elegans worm is that it develops the same each time (not all amimals do). The entire tree of cell divisions that creates C. elegans 1,000 cells has been constructed. In a corridor of MIT I once saw this tree posted where it ran about 20 feet down the corridor. The nervous system has only 302 neurons.
Estimating cell size from c. elegans worm
Note, eukaryotic
cell sizes vary over a huge range (1 m -1 um, or 10^6 by one estimate),
so there is bound to be uncertainty about how big an average cell
is. We can, however, use c.elegans worm to estimate the size of an average
cell, because it is a real animal with different cell types. Its 1,000
total cells can be proportioned as 25 x sq rt (40) = 6.3. A body length/width
of 4 which seems reasonable for a little worm, so our estimate is
(av) cell diameter = 1 mm/25 = 40 um
How did we do? Pretty well.
25 um --- (first cell reference above)
How does DNA unwind?
Virtually
every discussion of DNA replication that I have ever seen simply says that
DNA 'unwinds'. Think about it. DNA is a fantastically long twisted helix,
million (or billions) of twists. [Notice in the artist sketch below there
is one twist for every dozen or so bases.] How the hell can DNA just
unwind? This has long driven me nuts, and I am amazed that this issue
is never commented on. Even if biologist don't know, I would expect them
to address the problem and admit they don't know.
The first hint of an explanation I found was an excerpt from a book by Francis Crick. "There are special proteins whose job it is to unwind the double helix, together with others can put 'nicks' in the back bone to allow one chain to rotate around the other, and then join up the broken chains again." (Life Itself, Francis Crick, 1981). And I found this 2005 Science News announcement:
A Dutch led international team of researchers has unraveled how nature releases the torque built up in DNA at the molecular level. The researchers from Delft University of Technology, the Ecole Normale Supérieure in Paris and the Sloan-Kettering Institute in New York published their findings in the 31 March 2005 issue of Nature. An artistic impression of the enzyme at work is featured on the cover of this (Nature) issue.The enzyme topoisomerase IB releases the torsion built up in DNA strands. The topoisomerase clamps onto the DNA, cuts through one of the two DNA strands, and then lets the DNA unwind before sticking the broken ends back together again.
It sort of looks like Crick in 1981 knew such enzymes had to exist, but only recently (24 years later) have the enzymes been identified, and how they work is beginning to be understood.
Thomas Gold's speculations of cell evolution
At the end
of Gold's book, Deep Hot Biosphere, he throws in some speculations
on cells and cell evolution, which like everything Gold writes, are interesting
and could very well be right. He points out that the evolutionary mechanism
of change by the accumulation of useful random variations should work statistically
much better in microbes than in animals because microbes exist in enormous
quantities and reproduce quickly. Comparing a quickly reproducing microbe
to an elephant he calculates the microbes can have as much as a 10^22 adaptive
advantage. So it is not surprising, he says, that only microbes have developed
a wide variety of different biochemical pathways for extracting and processing
energy.
Another speculation Gold has is that the evolutionary development of animals and plants may depend on there being a means for plants and animals to acquire (in one gulp) microbial (hard won) biochemical machinery. For example, this could take the form of DNA gene splicing where a gene (or groups of genes) move from microbes to animals or plants, allowing whole biochemical pathways to be acquired. Gene swapping is now known to be common among microbes and is an important evolutionary mechanism, for example greatly speeding the acquisition of drug resistance in disease germs.
Another example, which has been advanced by Lynn Margulis of BU and is now pretty well accepted, is that entire microbes (in a kind of extreme symbiosis, called endosymbiosis) were once taken up now live (in a modified form) inside eukaryotic cells. Called organelles (an organelle is to the cell what an organ is to the body), they live inside cells with their own cell walls and their own DNA. Examples are mitochondria and chloroplasts (type of plastid) which contain the photosynthesis machinery of plants and algae.
http://en.wikipedia.org/wiki/Endosymbiotic_theory
Virus genes in human DNA
[Viruses to
cells] are like [memory chips to a computer]. Viruses are basically just
DNA that function by getting the cells protein making machinery to read
them to make the proteins specified by their DNA as well as making copies
of them. Retroviruses are single strand RNA. They work by first using an
enzyme (reverse transcriptase) to get themselves transcribed into a cell's
DNA, and then cells transcribe their genes into proteins in the usual way.
If the cell that a retrovirus inserts itself is an egg or sperm cell, then
the retrovirus' genes can get become incorporated into the cell line and
will be passed to future generations.
According to recent stories in Scientific American and NYT the current estimate is that 8.3 percent of the human genome can be traced back to retrovirus infections. A big NYT science story (Jan 2010) is that the first fossil DNA remains of a real virus (not a retrovirus) have been found in the human genome, saying, "four segments of human DNA have been found (by accident) that clearly had descended from a borna virus gene." Since no one was looking for (normal DNA) virus genes in human DNA, some scientist think much more than 8.3% of the human genome may have derived from virus and retroviruses.
Calories to fat (7/19/13)
The rule of
thumb that dietary references quote relating calories to fat is:
1 lb fat <=> 3,500 calories
The justification of this can be 'derived' from the calorie value of foods. Consider: 1 gram of sugar and protein contains 4 calories and 1 gram of fat contain 9 calories, one pound is 454 grams, and fat in human body is estimated to be 87% lipid.
454 grams x 9 cal/gram x 0.87 lipid = 3,554 calorie
In other words the caloric content of one pound of fat on the human body is (about) 3,500 calories. From what I read the calorie value of fat is only an estimate and so too the lipid fraction of fat, so 3,500 is only approx true with maybe a +/- 25% tolerance.
Based on this fat/calorie relationship the medical 'fat' literature almost universally says that to lose a pound of weight (other criteria constant) requires a diet deficiency of 3,500 calories, and conversely an excess ingestion of 3,500 calories lead can (might?) lead to a weight gain of one pound. And following this rule they will say things like one (additional) 50 calorie biscuit/day can lead to an annual weight gain of 5 lb, since [356 x 50/3,500 = 5.2]. But do the fact bear this out? Is it true in the real world? No it's not!!
The NYT today reports on an experiment where lean identical twin were confined for 120 days so their activities and output could be monitored, and they were fed an excess of 1,000 calories/day. I haven't read the paper, but a simple interpretation would be that they ate an excess of 120,00 calories during the four months. Using the [1 lb fat <=> 3,500 calories] rule of thumb we would estimate that the maximum possible gain from this 120,000 calories would cause would be [120,000/3,500 = 34 lbs].
34 lbs turns out to be pretty close to the mark. The maximum weight gain (about same for both twins) during the experiment was 29 lbs, but some other twin pairs only gained about 10 lbs, only 1/3rd of the maximum gain. This 3:1 ratio of how humans respond to excess calories is typically of what experiments like this show.
Conclusion
The conclusion
appears to be that genetically some people are efficient in processing
food calories and nearly all the calorie value of excess food can end up
as fat in their bodies. For them the [1 lb fat <=> 3,500 calories]
is a pretty good guideline. But other people are either less efficient,
or more likely have mechanisms (not understood) that they can call on when
at their target weight, to not absorb about 2/3rd of the excess calories
consumed, with the result that their weight gain is only 1/3rd of what
the guideline would suggest.