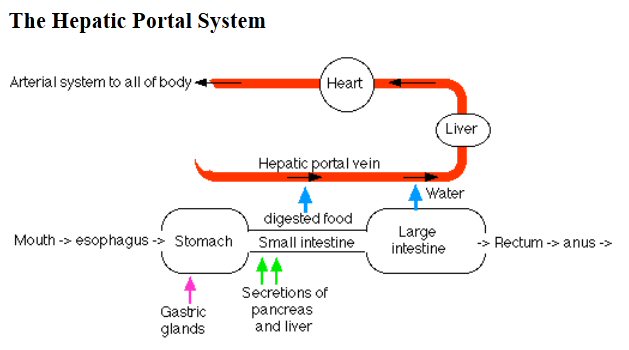
(source -- http://www.biology-pages.info/G/GITract.html)
Glucose overview
Sugar overview
Blood
sugar control mechanism
How much
food for 2,500 calories?
Essential amino acids
Understanding
the energy of food
What is food?
The human body, like plants, runs primarily on the sugar glucose, the king of sugars. A few respected scientists have begun to argue that non-glucose sugars, specifically fructose, the sugar in fruit & vegetables, can overload the liver leading to weight gain and diabetes. A fad? I always tend to be skeptical of fads, especially in food where faddishness seems to run riot. Are the 'fat is bad' people now just moving on to 'sugar is bad'? I set out to look into this.
Glucose overview
All plants,
and photosynthetic bacteria too, live on the sugar glucose. Photosynthesis
basically makes only one thing: glucose (C6H12O6), which is a sugar
and a carbohydrate. Plant cells burn glucose for energy, loosely link up
glucose molecules into starch for energy storage, and tightly link up glucose
molecules to provide structural molecules (think cellulose) to build the
plant.
Humans too basically live on glucose. Glucose is the sugar in the blood. And via the blood glucose is the main energy source for every single cell in the body. The body loosely links up glucose into glycogen for energy storage in muscles and in the liver for energy between meals. All sugars in the diet are quickly cleaved into simple sugars, and non-glucose simple sugars (fructose and galactose) are converted by the liver to glucose or its stored form glycogen. Table sugar (sucrose) is half glucose, the other half fructose. Fructose is the dominant sugar in fruit juice. The sugar in milk (lactose) is half glucose, the other half galactose.
Intro
A long article
in the NYT magazine in April 2011 'Is Sugar Toxic' by Gary Taubes (health
journalist) discussed how various sugars are processed by the human body.
A currently hot popular topic is that the biological effects of different
types of sugar are not the same, and the article laid out the circumstantial
case that consumption of too much sugar in recent decades, partly due to
high fructose corn syrup being in everything, is responsible for people
getting fat, diabetes, etc.
http://www.nytimes.com/2011/04/17/magazine/mag-17Sugar-t.html?_r=1&scp=3&sq=sugar%20taubes&st=cse
Taubes starts by talking extensively about a 90 min talk by U of C professor Robert Lustig who argues that sugar is a toxic chronic poison. Taubes says he wouldn't be writing the NYT article if he didn't also believe this, but this is an understatement.
Taubes is anti-sugar/carbohydrate crusader
A check of Taubes web site shows that he pushes an unusual, unbalanced diet: high fat, low sugar, basically he lives on eggs and steak, he is not a neutral observer. He eats his cheesburgers without a bun to avoid the starch! He posted this summary of how he eats:
"Keep in mind as you go through these that I do indeed eat three eggs with cheese, bacon and sausage for breakfast every morning, typically a couple of cheeseburgers (no bun) or a roast chicken for lunch, and more often than not, a ribeye or New York steak (grass fed) for dinner, usually in the neighborhood of a pound of meat. I cook with butter and, occasionally, olive oil (the sausages). My snacks run to cheese and almonds. So lots of fat and saturated fat and very little carbohydrates. A deadly diet, according to Dr. Oz." (http://www.garytaubes.com/blog/) (4/18/11)Here is the Lustig video on YouTube (over 1 million hits). I listened to the whole 1.5 hr (in two sittings). As Taubes himself says, the world to Lustig is black and white.
http://www.youtube.com/watch?v=dBnniua6-oM
(update)
Of course, a couple
of week after Taubes article a follow up article in the NYT quoted various
experts as saying that for those "in motion" drinking lots of fructose
may not be a bad thing. In an endurance sport the liver can deplete all
its glycogen stores. Tests show (not surprisingly) that in this state,
if you drink a lot of fructose, it restores the liver's glycogen levels
much more quickly than drinks with glucose.
Reading Taubes article I realized I knew next to nothing about sugar. I had no idea what fructose, sucrose, or dextrose were, what types of sugar are in table sugar and fruit juice (which I drink a lot of), how sugars are used and stored, and what is the role of insulin in the metabolism of sugar. Some background knowledge like this is necessay to even to begin to approach the 'sugar is toxic' theory.
From my work on photosynthesis I was familiar with glucose (C6H12O6). This sugar is the primary end product of photosynthesis and is what all plants and photosynthetic bacterial live on. So I decided to learn a little something about sugar, writing this essay in the process, then to reread Taubes, listen to Lustig, and critically evaluate the case being put forward by them and others.
Sugar overview
At first the
discussion of sugars seems complicated. There are at least a half dozen
sugars (single sugars, double sugars) all with similar sounding names:
glucose, fructose, sucrose, maltose, etc, but after I dug into it for a
few days, the veil lifted.
Simple sugars
The first simplification
is the recognition that there are only three simple sugars (of any
significance): glucose, fructose and galactose. All other sugars (table
sugar, fruit sugar, milk sugar) are combinations of these three simple
sugars. Double sugars are split up by enzymes in the stomach and small
intestine, so the only sugars that get absorbed into the body (from the
gut) are simple sugars.
All three simple sugars are structually close and have exactly the same chemical formula (C6H12O6), so they all have exactly the same number of calories. You may have heard that a 'calorie is a calorie'. So does that mean all these simple sugars are equivalent in the body? Well, not really. It turns out the body's main energy supply is glucose, so glucose is processed inside the body very differently from fructose and galactose.
Glucose is king
Just as with plants
the key #1 sugar in the animal body is glucose (C6H12O6). This is
the sugar that all the cells in the body burn for energy. It's the
sugar that's in the blood. It is the 'food' that the cells' energy factory,
the organelle mitochondria, turn into ATP and NAD+ for the cell's protein
machinery to use. Virtually every cell in every creature (animal, plant,
bacteria) uses glucose as its primary energy source. It's metabolism equation
(written in summary form) is just the opposite of photosynthesis equation.
During metamolism its carbon and hydrogen join with oxygen from the air
(or water) to form CO2 and H2O. The movement of the H and C valence electrons
into the deeper energy well of O releases energy. Every gram of sugar (or
protein) metabolized releases 4 'food' calories (1 food calorie =
1 kcal).
Glucose storage
Glucose is stored
in two places in the body: liver and in muscle cells. Glucose is soluble
in water, so in its native form glucose is not easily concentrated (would
cause osmosis problems) or stored. The glucose storage problem is solved
by enzymes in the liver and muscle cells that chain (and can unchain) the
small gluose molecules together into huge molecules (weight 30,000) called
glycogen, which are not soluble. (Runners are said to run on their glycogen
reserves.) In plants glucose is stored as starch. Plant starch in the diet
is broken down by the body converting it back to glucose.
Fructose & galactose
The other two (major)
simple sugars are fructose (fruit and vegetable sugar) and galactose
(milk sugar). They both get converted (by efficient biochemical machinery)
into glucose or its stored form glycogen, after they exit the small intestine
and enter the liver. Mostly they get converted to glycogen for storage
in the liver. In contrast most glucose (80% says Lustig) bypasses the liver
going directly into the blood stream, either for immediate use by the cells
or for storage (as glycogen) in muscle cells. Of course glucose is continually
being pulled from the blood by all the cells of the body burning it, so
between meals the liver slowly reconverts ('eats') its glycogen stores
and sends glucose out into the blood stream.
Double sugars
Simple sugars do
exist in the diet (in fruit mostly), but most sugar consumed is a double
sugar. Double sugar also arises (as an intermediate) when the body metabolizes
starch in the diet. Double sugars are just two simple sugar molecules connected
by a single bond. The simple sugars pair up in various combinations and
each, of course, has its own chemical properties and its own name.
For example, table sugar or sucrose (from sugar cane or sugar beets) is [glucose + fructose], lactose (from milk) is [glucose + galactose], maltose (from disassembled starch and beer brewing) is [glucose + glucose]. The first thing the body does with double sugars is cut the single bond between them (using enzymes in stomach or small intestine), so they enter the liver the same as if they had been eaten as free simple sugars.
'A calorie is a calorie'?
The 'sugar is toxic'
argument focuses on work load on the liver of processing non-glucose simple
sugars. The critics say it is not true that in diet 'a calorie is
a calorie', 'one sugar is not the same as another', because the liver based
processing of fructose and galactose is very different from the (whole
body + muscles + liver) processing of glucose.
Why focus on fructose?
Curiously the 'sugar
is bad' argument (by Lustig) focuses almost entirely on fructose. Galactose,
which also has to be processed by the liver, is barely (or never) mentioned.
Glucose is seen as the natural sugar, the sugar we evolved to eat. Fructose
and galactose are both processed in the liver in what seem to me to be
similar ways. So why is the focus only on fructose? My guess is
it's because there is (probably) a lot more fructose in the diet than galactose,
since the only significant source of galactose is milk. Also fructose consumption
continues to rise while consumption of galactose (milk) is probably either
flat or falling. I suppose it's possible though that the biochemists see
less risk in overload in the liver when it's processing galactose.
On sugar --- glucose, fructose, etc
Lustig knows
a lot of biochemistry and lays out the mechanisms for the damage sugar
can do. His focus is fructose and how it is metabolized, in the liver,
where it can easily get converted to fat. However, on page 3 (of 10) of
Taubes article an outside expert says the real issue with the danger of
fructose, like with any poison, is dose. Yup. Here is where Lustig
is very weak. Sure he gives examples with number, like drinking a 120 calorie
drink and following the calories through the liver, but that is not the
point. The key issue is, at what dose and/or dose rate (!) does sugar/fructose
go from being a simple calorie food to being (possibly) toxic, from being
easily handled by the liver to overloading the liver.
Lustig also blithy dismisses a famous graph from 30 years ago showing heart disease rising (almost linearly) with (saturated?) fat in the diet. He says Ancel Keys (Univ Minn) did his analysis wrong. Keys studied 7 countries and concluded that heart disease went up as high serum cholesterol levels went up brought on by a diet high in saturated fat.Simple sugars (monosaccharides)Lustig says his regression analysis was wrong (or not done) and that sugar also rose with fat, so what the graph really shows is heart disease rising with sugar consumption in the diet. I have no idea of the science behind this argument, but he presents it as settled fact, and it's a little too neat, too tidy, to have your opposition make your point for you.
Researching this I find there has been a lot of debunking of the cholesterol & saturated fat in diet and Ancel Keys work too. On reference is the book 'The Great Cholesterol Con' by Anthony Colpo (50 Amazon reviews, 4.5 stars) I remember reading years ago (in the Atlantic?) a devastating critique showing that all the large scale low cholesterol diet studies had failed to extend life.
In terms of sweetness, fructose is (by far) most sweet, then glucose, then galactose. All have straight 6C chains and looped back forms. All have the same chemical formula. In chain form from they are pretty similar, but with some structural difference in the chain center. For example I read of ribose that "all the hydroxyl groups (OH) on the same side in the Fischer projection".
glucose
C6H12O6 basic sugar, 'burned'
in all cells for energy, the primary product
of photosynthesis, blood sugar. Since all sugars used
in life are right handed, 'dextrose' (right handed glucose)
is just an alternate name for glucose.
fructose
C6H12O6 also called fruit
sugar
galactose
C6H12O6 part of the double
sugar in milk (lactose)
ribose C5H10O5 (a sugar, but not really food, it's biochemical, core of ATP)
Glucose is 'blood sugar' and is the primary metabolic fuel for humans. It is more stable than galactose. Ribose is part of ATP. ATP is made up of the nitrogenous base adenine, the five-carbon sugar ribose and three phosphate groups.
Double sugars (disaccharides)
table sugar
or sucrose
50% fructose/50% glucose (loosely connected,
C12H22O11
both ringed)
high fructose
corn syrup
55% fructose/45% glucose (biochemically the
same as sucrose (table sugar))
lactose 50% galactose/50% glucose (sugar in milk)
Generated in the intestine from plant starch
maltose
double glucose (plant starch is broken down
to glucose and maltose)
Sucrose (plus free glucose and fructose) is fruit and vegetable sugar. A table in Wikipedia ('fructose') shows that most fruits, like apple, have more free glucose and free fructose than the paired double sugar sucrose. At the other pole are the vegetables sugar cane and sugar beets. They are unusual in that virtually all their sugar (which is high) is in the form of sucrose. Table sugar is little more their sugar filtered and dried. Lactose is milk sugar.
Any number of glucose can join in a string, and all the strings have names. Two glucose is maltose, three is maltotriose (trisaccharide), etc.
Triple sugars
There's a
cool triple sugar (trisaccharide) called raffinose. It is all three
simple sugars combined [glucose + fructose + galactose]. It's found in
vegetables beans, cabbage, etc.
The other triple sugar I see mentioned frequently is maltotriose [glucose + glucose + glucose]. It forms in the body when starch (stored plant glucose) is digested.
Separating double sugars
While in principal
double sugars can be separated by water (hydrolysis) or by acid in practice
these reactions are very slow. I read hydrolysis can take years and separation
of two glucose (maltose) with hot strong acid takes several minutes. In
practice the separating (& joining) of sugars is done in the body with
specialized enzymes that do the job quickly.
Glucose vs fructose
These two
'simple' sugars have exactly the same formula (galactose has same formula
too). Both exist as a straight 6 carbon chain and in a looped back form.
As I compare their structures, this is what I see. The ends in the glucose
chain are not the same: one end is CH2OH (which is C coupled to O + 2H2
with the 3rd H on the O).
The fructose chain is quite similar to glucose, but has some rearrangements. Fructose has symmetrical ends, both CH2OH. Like glucose it has one double bonded O, but whereas in glucose the double bonded O is at the end (#6 position) in fructose it is at the #5 position. Because the double bonded O is one back, when fructose loops back it froms a smaller (five sided) loop [4C +O].
Galactose
Galactose
comes mostly from milk (and milk products) whose sugar (lactose) is half
galactose and half glucose. Enzymes pull it apart, but I am having a hard
time finding out where those enzymes are located. Breast milk sugar is
in the form of lactose, so new mothers either need a source of galactose
or the ability to make it.
Galactose can be converted to gluocse (in liver?) and apparently galactose also circulates in blood, because human breasts pull it from blood and use it to make lactose for breast milk. The human breast contains enzymes for converting glucose to galactose for making lactose.
No references says this, but it makes sense to have a backup because you can't have breast milk manufacture dependent on the mother having recently drunk milk! So it's a double system, if galactose is available in the blood, it will be pulled out and used, but if not it can be manfactured. Wikipedia says typically 2/3rd of galactose in breast milk comes from the blood and 1/3rd is manufactured, but seems to me this must be very variable.The other side of the coin (reading between the lines) is that if breast milk is not being made there must be biochemical machinery to use galactose, a primary sugar, and major ingredient in milk. This must consist of the enzymes to separate lactose (into glucose and galactose) and enzymes to convert galactose to glucose.
From a technical paper on Leloir pathwayMore reading shows galactose has various uses in the body (used structurally in cell walls, for example) and that the human body manufactures a few grams of it every day.
The processing of galactose is done by the Leloir pathway (named for guy who figured it out) and involves ecoli bacteria, so my guess is it happens in the gut. Ecoli "encodes" the enzymes, which I guess means ecoli bacteria provide the enzymes needed to cut lactose to galactose and glucose. Galactose after processing is "an efficient source for glycogen biosynthesis as glucose itself." The implication is that the sugar glactose can be used (like fructose) in the liver to make glyocen (linked gluose molecules) energy stores.
Blood types
Turns out
a major difference in blood types (A, B, O) is in the (simple) sugars they
contain. All contain some fructose and galactose along with glucose, but
B type contains more than O or A.
Sugar processing
Table sugar
(sucrose) is taken apart (by enzymes) into glucose and fructose by the
first step in the digestive process, the stomach. Glucose and fructose
then pass into the blood via absorption in the small intestine, but this
blood flow is somewhat separate from the general circulation system because
it flows (via a special pathway) directly to the liver.
Intestine to liver
I learned
something interesting about how the liver processes food. There's a special,
short (3 inch) path from the intestines to the liver. This is important
because all sugars except for glucose must be converted by the liver.

(source -- http://www.biology-pages.info/G/GITract.html)
How it works is this: The vein that drains blood from the intestines flows directly to capillary beds in the liver. It's called the hepatic portal vein. The blood coming from the intestines carries absorbed food molecules like sugars. This direct path to the liver allows the liver to pull off the fructose for processing and let the glucose pass to the rest of the body. (Wikipedia says the hepatic portal vein is not a 'real' vein because it doesn't go (directly) back to the heart, but this sounds like a quibble. There are two other so-called "portal" system in the circulation system, one in the brain (between hypothalamus and pituitary), and the other in the kidney.) Sketches show the liver is high up in the abdominal cavity (in the back toward the right).
Small intestine
The surface
of the small intestine is lined with a forest of tiny vertical cells (microvilli
also known as a 'brush border') that hugely increase its surface area.
Here is where sugars, fats and other foods are absorbed and broken down.
On the membranes of the microvilli are enzymes that separate the various
double sugars (disaccharidases) into their constituent simple sugars glucose,
fructose and galactose.
Another interesting fact I learned is that the small intestine, where foods are absorbed, is basically sterile. It is the large intestine that is home to the fauna and flora of the gut, 100 trillion bacteria x10 more creatures than there are cells in the human body! What enters the large intestine (from the small intestine) is mostly water, which the large intestine extracts and sends to the bladder, and bits that are indigestible. One reference says this:
"Bacteria flourish to such an extent (in the large intestine) that as much as 50% of the dry weight of the feces may consist of bacterial cells!" So this is what shit is... And it goes a long way toward explaining why it basically always looks the same.In the liver
His glucose example --- Two pieces of white bread (120 calories of glucose). (Actually white bread is starch, which breaks down into glucose.) 80% of the glucose passes through the liver and goes directly into blood stream where it is used by all the cells of the body (for energy). Every bacteria, every living thing (he says) uses glucose for energy. It it the 'energy of life' (or something like this).
20% of the glucose coming into the liver is converted to chained up to form glycogen stores in the liver. He asks the question, "How much glyocgen can the liver hold?" and give the answer "unlimited" (he exacgerates!) using as proof people with a discease where they can't get the glycogen out of their livers and theivers get huge, but the livers don't fail.
His fructose example is a glass of orange juice (120 calories of sucrose, which is half glucose and half fructose). All the fructose is metabilized by the liver. "Only the liver can metabolize fructose." A fraction (30%) of fructose ends up as fat within the lever.
Sugar delivery
Bottom line:
Only one sugar, glucose, goes directly from the intestine into the blood
general circulation system for use by all body cells. The other sugars
must be processed (converted) in the liver either to glucose or to stored
glucose (glycogen) in the liver.
The references are a little unclear, but some galactose must (sometimes) also circulate in the blood. Wikipedia says some of the galactose the breast needs to make lactose for breast milk it obtains from the blood. My guess (an efficiency argument) is that galactose is only allowed to circulate in the blood of lactating females and that in all other cases the liver grabs the galactose coming from the intestine and converts it to glucose. (Or a variation of this would be galactose goes into circulation first, giving the breast a crack at it, with the liver slowly extracting it and converting it to glucose (or glycogen.)
Storage of sugar energy
The energy
demands of a lot of body cells are likely pretty constant. My guess is
that they are set up to pull what they need in terms of sugar for energy
from the blood. Then as long as the blood sugar level remain within normal
bounds they are happy. However, the energy demands of cells like muscle
cells (& maybe the heart) are obviously extremely variable, so it is
not too surprising that each muscle cell has within it its own reserve
of sugar for energy. The reserve of sugar energy for the body as a whole
is centralized and held within the liver? to be distributed by the blood
when needed.
Glucose is soluble -- can't be concentrated
A big problem with
storing sugar (glucose) in a cell (basically a bag of water) is that (raw)
glucose can't be concentrated because its soluble in water. The
solution to this problem that plants and animals have developed are specialized
enzymes that hook (& unhook) the small glucose molecules into long
loosely interconnected chains that are insoluble. These long glucose chains
aggregate into very large molecules forming particles (granules) that can
be seen in the cell. With the sugar molecules pulled out of solution the
osmotistic levels and pressure of a cell are not affected.
Animals store glucose in glycogen (plants in starch)
Interestingly
plants and animals do glucose chaining and storage differently. Plants
form the chained glucose into starch, while animals form the chained
glucose into
glycogen, sometimes called 'animal starch'. Glycogen
molecules are huge, typically 30,000 (!) glucose molecules hooked up to
form long tree-like chains branching from a central protein core. Unlike
with structural molecules like cellulose where glucose are tightly interconnected,
here the chains are pretty much linear and only loosely connected. To recover
the glucose for energy a dedicated enzyme pops them off the ends of the
many chains making up a glycogen molecule.
Starch
Starch is
just chained glucose molecules. Starch (polysaccharide) is produced by
all green plants (not animal cells) as an energy store. It is the most
common carbohydrate in the human diet and is contained in large amounts
in such staple foods as potatoes, wheat, maize (corn), rice, and cassava.
Blood
sugar control mechanism
The level
of blood sugar (glucose) needs to be regulated. Too much blood sugar causes
an acidic condition which damages organs and is the disease diabetes. The
level of sugar in the blood automatically rises after eating sugar containing
foods as it is absorbed into the blood from the small intestine. The sensor
for blood sugar level is (apparently) in the pancreas. As blood sugar levels
go up, the pancreas responds by putting more insulin (hormone) into the
blood.
The central storage location for sugar (as glycogen) in the body is in the liver. Muscle cells also pull glucose from the blood and store it locally (as glycogen) for their own use. Both the liver and muscle cells respond to insulin using it as the control signal to pull glucose from the blood and link it up into glycogen, where it stores as little granules.
Insulin is a signaling protein used to 'reduce' blood glucose levels (update 11/5/11)Simple sugar mechanism?
Insulin activates muscles and liver to absorb glucose After eating, blood glucose levels naturally rise as sugar and broken down food protein enter the blood. The pancreas is the controller for blood sugar working (apparently) as a linear controller. When glucose goes above the pancreatic set point, the pancreas begins to output the protein insulin. Insulin activates muscle cells and the liver to absorb glucose from the blood and store it away locally as glycogen. When insulin levels are low, below an insulin set point in the muscles and liver, the muscles and liver begin to break down their glycogen stores and release its glucose back into the blood to feed the cells of the body.The figure below shows that insulin levels vary during the day about five to one. Glucose levels in the blood are held to about a +/- (20% to 25%) range about a set point. That this is a linear control loop is shown by the figure, which shows that with the right setting of scale offsets and gains that daily curves of of insulin and glucose are very similar.
Note this picture immediately shows two ways this control loop can fail, and these are the two major types of diabetes. In one type of diabetes (Type 1) the mechanism for insulin release in the pancreas is broken. In the other major type of diabetes (Type 2) the mechanism in muscles and/or liver for them to respond to insulin levels (by pulling and releasing glucose) is broken.
Since insulin is a blood glucose reducer, a failure of the control loop results in blood sugar levels that are too high. The classic symptoms of diabetes are frequent urination, increased thirst and increased hunger.
a) Pancreas as regulator --- It senses blood glucose levels (presumably comparing it against a built in reference), and if blood levels are too high it outputs (more) insulin to signal the liver to release less of its glycogen stores into the blood.
b) Liver is being regulated --- The liver converts non-glucose sugars to glycogen for later release into the blood as glucose and presumably the level of insulin controls how it breaks down its glycogen stores for release to the blood.
So the overall (simple ) mechanism would be, if glucose levels are too high, the pancreas emits more insulin which causes the liver to output less glucose.
Double sugar mechanism
But
then I read this (Wiki -- Insulin resistance)
-- Certain cell types such as fat and muscle cells require insulin to absorb glucose. When these cells fail to respond adequately to circulating insulin, blood glucose levels rise. The liver helps regulate glucose levels by reducing its secretion of glucose in the presence of insulin.So it's really a double mechanism. The pancreas in outputting more insulin not only causes the liver to output less glucose (from its glycogen stores), but it causes the cells that maintain their own local energy stores (fat and muscle cells) to absorb more glucose and to store it away. (From another point of view the action of insulin is simpler. More insulin causes any body cell that can make glycogen (liver or muscle) to do so.) Presumably there is little that can be done with all the other body cells that automatically burn glucose based on their and the body's metabolic needs.-- This normal reduction in the liverís glucose production may not occur in people with insulin resistance. Insulin resistance in muscle and fat cells reduces glucose uptake (and so local storage of glucose as glycogen and triglycerides, respectively).
So to avoid diabetes not only does the pancreas need to work, but the liver, fat, and muscle cells need to respond properly to insulin in their intake or outputting of glucose. Weakness of the liver, fat cells or muscle cells in their to insulin will result in the pancreas increasing the insulin blood levels to get the desired effect.
Triple mechanism
If fat levels
in the blood are considered, insulin becomes part of a triple mechanism.
-- Other functions of insulin can also be affected. For example, insulin resistance in fat cells reduces the normal effects of insulin on lipids and results in reduced uptake of circulating lipids and increased hydrolysis of stored triglycerides. Increased mobilization of stored lipids in these cells elevates free fatty acids in the blood plasma.
Translation of above --- Insulin also controls how fat cells pull liquid fat (lipids) from the blood. If fat cells are responding poorly to insulin, then the lipid level in the blood rises.
Insulin & glucagon
While insulin
is the well known blood sugar controller, I read in Wikipedia that it is
a double system with two signalling hormones: insulin & glucagon. Blood
sugar levels are sensed and controlled by the pancreas. When blood sugar
levels rise after a meal (above a set point), the pancreas outputs more
insulin
(less glucagon). When blood sugar levels fall (below a set point), the
pancrease outputs more glucagon (less insulin).
Insulin and glucagon from the pancreas 'controls blood sugar'. Insulin stimulates an enzyme (glucose synthase) in the liver and muscles that gathers up glucose molecules and adding them to the glycogen chains to build up glycogen stores. Between meals the insulin level falls and the glucagon level rises. The liver responds by breaks apart its stored glycogen granules and releasing the glucose back into the blood to hold the blood sugar in the comfort zone for use by all the other body cells for energy.
High insulin
High insulin levels force blood sugar down. High insulin levels signal muscle and liver cells to absorb more glucose from blood (more than needed for immediate energy needs) and to store it away as glycogen.
High glucagon (low insulin)The figure below shows glucose (during the day) is held within about a x1.5 band (4 to 6 mmol/L). The insulin control loop is a little strange because the insulin level (around a glucose set point of about 5 mmol/L) responds quickly and in a linear way to glucose levels. The non-linear response (or offset) to the insulin control signal, whether to make more glycogen or to tear it down, must reside in the insulin response sensors in the liver and muscle cells.
High glucagon levels force blood sugar up. High glucagon signals the liver to break down its stored glycogen and to release glucose into the blood. (Glcogen stored in muscle cells is never released back to the blood.) Low insulin may also signal some (which?) cells to reduce their use of glucose.
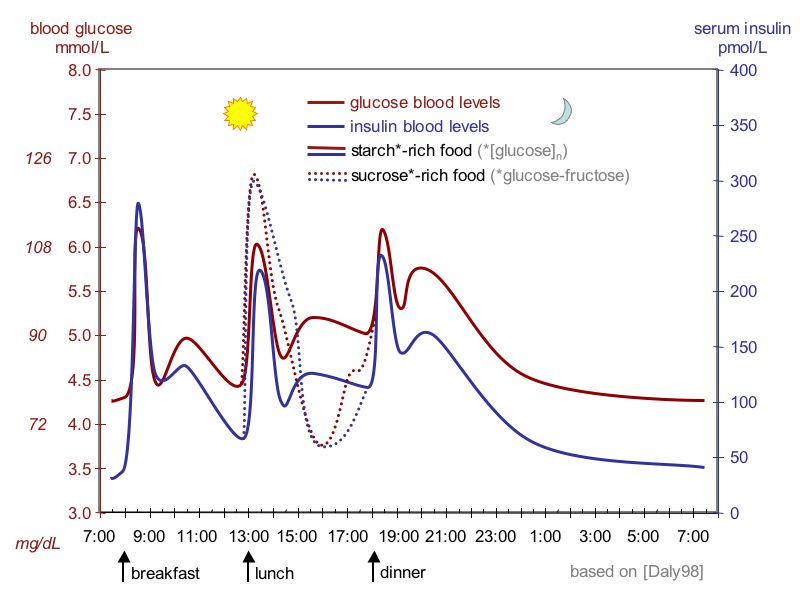
Note the left side glucose scale has an offset
(center about 5 mmol/L,
while there is s no offset in the right side insulin
scale
(source -- Wikipedia 'Insulin')
Glucose & insulin response times
From the above
graph it can be seen the 'pulse' glucose rise in the blood takes only 5-10
minutes after eating with the insulin rise following so fast it's not really
readable here. The liver and muscles (with their additional uptake of glycogen)
pulls down the 'pulse' of glucose in blood in about an hour.
-- Insulin is provided within the body in a constant proportion to remove excess glucose from the blood, which otherwise would be toxic.
Gluconeogenesis
The body has
a backup mechanism (called gluconeogenesis) to keep blood glucose levels
from falling too low in case of fasting, low carbohydrate diets, etc. If
the liver runs out of stored glycogen, then it has the biochemical machinery
(kidney can do this too) to convert some fats (lipids) into glucose (for
the blood). Gluconeogenesis cycle can make glucose out of proteins too.
This allows the body to consume itself during starvation. The Wikipedia
article is little vague as the source of the fats, apparently because this
process is not fully understood, but the main source apparently is lipids
from fat tissue (adipose tissue) that come to the liver for conversion
to glucose.
Bottom line --- Glucose, as the primary energy source for all cells and a regulated blood component, can be made from almost anything (fats, proteins, or other sugars) in food or the body itself.
-- Adipose tissue or body fat or fat depot or just fat is loose connective tissue composed of adipocytes. It is technically composed of roughly only 80% fat; fat in its solitary state exists in the liver and muscles. Adipose tissue is derived from lipoblasts. Its main role is to store energy in the form of lipids.
What about the low sugar/carbohydrate (high fat) diet?
Maybe I am
missing something but it seems to me low sugar/glucose (high fat) diet
that Taubes is on increases the load on the liver. If you don't eat hardly
any glucose, or carbohydrate that will break down into glucose, where does
the glucose all your body cells need to live on going to come from? The
answer must be (I presume) the liver. The liver will need to process the
proteins and fat in the diet into glucose to keep blood sugar levels up.
If the objective were to give the liver the lightest load, then shouldn't the diet contain just the amount of glucose (or carbohydrate equivalent when broken down) the body uses daily, not more not less and with the minimum of other sugars?
Are Taubes and Lustig on the same page? Lustig goes on and on that too much fructose is overloading the liver. But if you don't eat enough glucose (or carbohydrate equivalent) for the glucose you body cells pull from the blood, then the liver has got to make this glucose from what you are eating. Seems to me this diet increases the load on the liver, just in a different way than processing fructose or galactose does.
Liver can't handle fructose?
Or is the main thrust
of Lustig's argument that the liver is particularly bad at processing fructose?
That even though fructose and glucose have exactly the same number and
type of atoms the biochemistry shows the liver can too easily (if overloaded?)
convert fructose into liver fat. An interesing question is: 'Do fat people
have really fatty livers?' Mixed in too seems to be the claim that fructose
screws up the insulin signal and fat cells (?) get confused.
So what are
these advocates saying? Is the focus of their diet 'low sugar and
low starch' (low carbohydrate), or is it 'low fructose'?
------------------------------------------------------------------------------------------------------------------
How
much food for 2,500 calories?
One way to
figure how much protein, fats, and carbohydrates (sugars) in a diet is
to start with their calorie content per gram. One hospital's very low calorie,
low carbohydrate diet was 1,000 calories per day, high in fat and limiting
carbohydrates to just 40 food calories (4%), equivalent to 10 grams daily
of carbohydrates, or 4 kcal/gram. Caloriies per gram for various food types
(below) can be figured from any USDS food label (food cal = kcal):
fat
9 cal/g (38kj/g)
carbohydrate (sugar) 4 cal/g
protein
4 cal/g
For a baseline 2,500 calorie diet (for men)
100% fat
278 grams (0.61 lb, 9.8 oz)
100% protein or carbohydrate
625 grams (1.38 lbs, 22.1 oz)
Balanced diet
33% calories fat
93 grams (0.20 lbs, 3.3 oz)
67% calories protein & carbohydrate
417 grams (0.92 lbs, 14.7 oz)
-----------------------------------------
510 grams (1.12 lbs, 17.9 oz)
Recommended levels of fat (by USDS) for a 2,500 calorie diet is 95 grams (0.21 lb), which is [95 grams x 9 cal/g] = 855 calories, or 34%. There must, of course, be a utilization factor, but the implication of the food labels is that it is somehow built in.
Of course this doesn't include the weight of water in the food. One cup of apple juice has 120 calories, so dissolved in its approx 240 grams of water (240 ml) is 30 grams (= 120 cal/4 cal/gram) of sugar (2/3rd fructose). While by weight apple juice is approx 1/8th sugar, each sugar molecule (C6H12O6) weight approx x10 water molecule, so (in round number) only about 1% of the molecules of apple juice are sugar molecules.
-- The differing energy density of foods (fat, alcohols, carbohydrates and proteins) lies in their varying proportions of oxidizable carbon atoms. Release of energy from food follows transfer of electrons from carbon and hydrogen to oxygen (carbon dioxide and water).
How much oxygen?
Fats have
more than x2 energy per weight because they don't contain much oxygen.
Here a quick calculation using atomic weights [H=1, C=12, O=16] of the
fraction by weight of non-oxygen atoms (C & H) in a fat and a sugar.
fatty acid (triglyceride) C55H98O6
(55 x 12 + 98)/(55 x 12 + 98 + 6 x 16) = 758/856 = 89%
sugar (carbohydrate)
C6H12O6
(6 x 12 + 12)/(6 x 12 + 12 + 6 x 16) = 84/180 = 47%
Much of the higher energy per gram of fat (9/4 = x2.25) is explained by the fact that only a small fraction of its weight is oxygen (11% vs 53% for sugar), so it has almost twice (x1.89) the number of carbon and hydrogen atoms per unit weight.
Another important consideration is that a quite a few of the carbon and hydrogen bonds in a sugar (compared to a fat) are already with oxygen, so these will not contribute to net energy output when remade in CO2 and H2O. One quarter of the valence bonds in sugar are oxygen bonds [(6 x 2)/(6 x 2 + 12 x 1 + 6 x 4)] = 12/48 = 0.25 (25%). There are no O=O bonds in sugar, so all of the O bonds in sugar are to C or H. Whereas fat with about 1/5th the fraction of oxygen atoms probably has roughly 5% of its bonds with O. These two factors I think explain the 225% energy advantage of fat.
Fruit juice
I drink a
lot of fruit juice. So how much fructose is this? Working assumption 2/3rd
of the sugar in fruit juice is fructose (it varies, but I remember this
ratio for apples). Mostly I drink just a little at a time as a snack, maybe
1/3rd cup (40 cal < 2% of daily calories), which I would think would
render any liver overload arguments moot.
I've checked
a lot of fruit juice labels, and it's amazing that almost any fruit juice
you pick up is 120 calories/(8 oz glass, a cup) essentially all from fruit
sugar. It's the same 120 calories for 100% juice and concoctions like limeade,
which is basically just high fructose corn syrup with a little juice. Half
gal jug has 8 cups, so round numbers, drinking one jug/week is (about one
cup/day, or 120 cal/day), about 5% of a 2,500 calorie diet. Not too bad.
Going through two half gallon jugs/week raises the fruit juice sugar in
the diet to 10%, still I would think well within the 1/3rd of a diet sugar/starch
should be.
---------------------------------------------------------------------------------------------------------------------------------------
Real world diet numbers
NYT article
give av weight lost in a study by fat people in 10 weeks on an extremely
low calorie regimen.
"Although some people dropped out of the study, most of the patients stuck with the extreme low-calorie diet, which consisted of special shakes called Optifast and two cups of low-starch vegetables, totaling just 500 to 550 calories a day for eight weeks. Ten weeks in, the dieters lost an average of 30 pounds." (NYT 12/28/2011)My first estimate numbers. Diet 1,200 cal short of normal daily needs. Food calories are 9 cal/gram for fat and 4 cal/gram for protein and carbohydrate. I guessed 6 calories net obtained from 1 gram pulled from fat storage. That means a daily loss of 1,200 cal/(6 cal/gm) = 200 grams (0.44 lb). In 70 days (10 weeks) this is a loss of 31 lbs. Lot of guesswork here, but it supports the idea that with a strict diet weight lost can be pretty well estimated from the calorie deficient.
Diet has a small effectThese guys always need a gimmick and Fuhrman likes to speak of a concept he came up with called, 'nutrient density'. He advocates eating foods with high nutrient density. He has a formula for it:
Years ago there was a big push to lower cholesterol by changing diet, doctors were advised to always recommend a diet change first, but when major clinical tests were run, it was found that trying to change cholesterol levels via diet was basically a waste of time, it doesn't work. The reason, of course, is that the body has built-in a cholesterol thermostat and the body can manufacture cholesterol as needed to keep the cholesterol level at its internal set point.Similarly a Dr. Dean Ornish, well credential and respected doctor (Prof of medicine at Univ of Calif), has for years been recommending, and in his case with some evidence, that heart disease can be reversed with diet. The problem is that for this to work the diet must be extreme, in his case super low fat (< 10% of diet calories from fat. Fat is where the flavor is!) which most people find very hard to live with.
Health = Nutrients/calories
Nutrients, of course, are only vaguely defined. He says most of the health protective components plants contain (what they are not in meat!) are unnamed. He uses the term 'phytochemicals, which I had never heard of, but has an article in Wikipedia.
"Phytochemicals are chemical compounds that occur naturally in plants. The term is generally used to refer to those chemicals that may affect health". (Wikipedia. emphasis in the original)Of course, a lot (maybe most) of those weird compounds in plants are not there to nourish animals and insects that eat them, but are protective chemicals to poison or otherwise discourage them from the plant. (Sure enough the first phytochemicals I clicked on in Wikipedia said it was thought to serve as a plant defense.) What's cool (to Fuhrman) about his 'nutrient density' ratio is that he can now assign numbers to various foods and then rank them as to their 'health' benefit! Here's his chart:
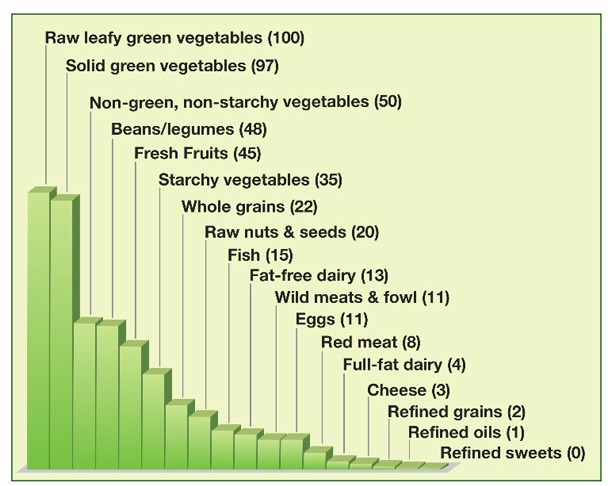
(source -- http://www.drfuhrman.com/library/article17.aspx)
Translation
What, of course,
the formula shows is that you should eat a high volume of crappy (low calorie)
food! This why meat is bad he says, too many calories per gram. Your food
should have low calorie content (per gram) to keep the denominator low.
The only evidence I have seem him give for this is a study showing that
high roughage food (plant food) passes through the intestine twice as fast
as high protein meat (with low roughage he says), and the longer travel
time through the intestine of meat leads to a doubling of colon cancer
(or so he says).
Refined sweets are 0!
Well I guess
it's true that sugar (C6H12O6) is only sugar. It provides in pure form
the energy (measured as calories) the body needs to maintain its temperature
and to move (do work). I guess from Fuhrman's point of view this is a bad
thing!
=========================================================================
Essential amino
acids
Proteins are
assembled from strings of 20 or so amino acids. Wikipedia (Amino Acid)
has a chart listing "21 amino acids found in eukaryotes". A little
less than half of them (about nine) cannot be synthesized by the body so
they must be included in the diet. These are the 'essential' amino acids.
The is some squishiness in coming up with a definitive list of 'essential'
amino acids. For example, two of the amino acids contain an atom of sulfur:
methionine and cysteine. Neither can be created 'de novo' as they say by
the body, but if you have one, it can be converted into the other.
I paged through all 21 amino acids in the chart and 18 of them are composed of just four atoms: carbon, hydrogen, oxygen and nitrogen. Two of the 21 have one sulfur atom and one (probably pretty rare) has one atom of selenium!
Still, it's surprising that references have different lists. For example the Univ of Arizona has a biology project on the Chemistry of Amino Acids.The history is that it was recognized that some amino acids were essential when it was found that rats could not survive on zein, which is a protein from corn, but recovered if casein, a milk protein was added to the diet. Turns out the zein diet was missing threonine. With more manipulation diet tests it was eventually shown that rats needed ten amino acids in their diets."The 10 amino acids that we can produce are alanine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine and tyrosine. Tyrosine is produced from phenylalanine, so if the diet is deficient in phenylalanine, tyrosine will be required as well. The essential amino acids are arginine (required for the young, but not for adults), histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine." (Univ of Arizona)Note neither 'arginine' nor 'histidine' appears in the essential amino acid table (below), which I got from Wikipedia ('Essential Amino Acids'). Checking the Wikiedia article 'histidine' I find it is there listed as an essential amino acid. It goes on to say it was originally thought to be necessary only in infants, but then references a 1975 paper where (normal) people fed a diet missing histidine developed blood problems. The Wikipedia article on 'arginine' says it is one of the most common amino acids, but it's status as essential is fuzzy -- "In mammals, arginine is classified as a semiessential or conditionally essential amino acid. Preterm infants are unable to synthesize or create arginine internally, making the amino acid nutritionally essential for them. In general, most people do not need to take arginine supplements because the body usually produces enough."
Tiny quantities of amino acids
Here is something
interesting I noticed. Wikipedia has a table (below) listing the quantity
needed daily of the essential amino acids. Notice how tiny the quantities
are! There are only 20 or so amino acids that form all proteins, yet the
quantity needed in the diet for most of these is only 1 or 2 grams!
An optimum mix (adding the number in the table) of essential amino acids
is about 12 grams out of what would typically be 210 grams of protein in
a 2,500 calorie diet (see below).
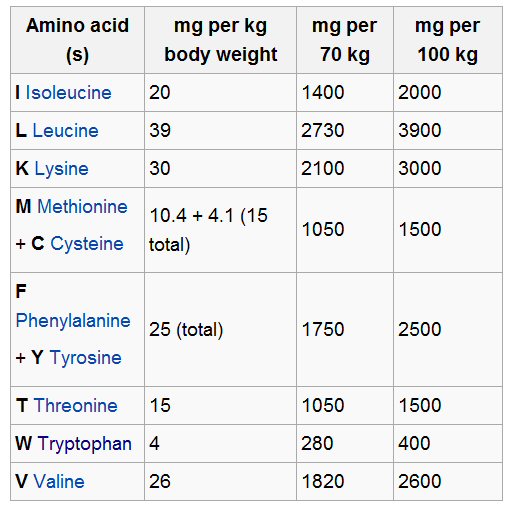
another Wikipedia article gives a value for limiting
amino acid lysine (840 mg) almost x3 lower than above
(source -- Wikipedia 'Essential Amino acids')
-- Although proteins from plant sources tend to have a relatively low biological value, in comparison to protein from eggs or milk, they are nevertheless "complete" in that they contain at least trace amounts of all of the amino acids that are essential in human nutrition.
-- Limiting amino acid in diet is usually lysine. This amino acid (with nitrogen) is low in cereal grains, but high in legumes.
-- A food is
considered to have sufficient lysine if it has at least 51 mg of lysine
per gram of protein (so that the protein is 5.1% lysine).
---------------------------------------
Alternate commercial sugars
Googling sugar
issues I occasionally stumble upon web sites that focus on alternates to
'refined sugar' (as though this was a problem). They list various sugar
substitutes.
Agave nectar
Mostly (90%)
fructose. From the agave plant used to make tequila in Mexico and South
Africa. This plant looks like a cactus, but its related to the asparagus,
so basically its a vegetable. The syrup is described as being sweeter than
sugar.
Maply syrup
Mostly sucrose.
Sucrose is table sugar (glucose + fructose). Sugar maple tree stores energy
as starch over winter and in spring converts the starch to sugar which
feeds the tree via sap.
Honey
Mostly fructose
(38%) and glucose (31%) . From bees (obviously)...
Brown rice syrup
Mostly maltose
(45%) and maltotriose (52%). Made from rice. Breaks down into glucose since
maltose is double glucose and maltotriose is a triple sugar consisting
of three glucose. As a triple sugar it takes longer to break down (2-3
hr) than double sugars.
----------------------------------------------------
-- Animals,
including humans, lack the necessary enzymatic machinery and so do not
synthesize glucose from lipids (fats)
Gut flora modify foods
How little
understood is the effect of diet is hi-lited by a recent article in Nature
(April 2011), which I stumbled on.
Differences
between people re: [relation between diet and heart disease] can depend
on the type of flora in the gut. A common food additive, lecithin (yellow-brownish
fat in egg yokes), also in mulit-vitamins, is found (via a newly discovered
pathway) to be converted into "harmful metabolites linked to heart disease"
by "gut microbiota" of some people (& mice).
=====================================================
Glucose forms
The textbook
structure of glucose is a 6 carbon linear chain. Carbons make four
bonds, so each inner carbon of the chain uses two of the four bonds to
form the chain. The two remaining bonds of the carbon along the chain are
taken up by an H and the O of an OH. Thus each inner carbon has the equation
CH2O, consistent with the formula for glucose (C6H12O6).
 .
.
Glucose -- six carbon chain
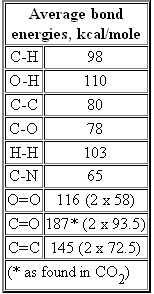
Bond energy in glucose (in kcal/mol)
Ring form
of glucose (see below) flips back the C=O end to join the 2nd carbon to
form a six-sided ring (5C + O). The oxygen that ends up in the ring is
not the #6 (right end) oxygen, but the #2 oxygen (former part of OH). The
(right) end carbon break one of its C=O bonds and this carbon joins with
the O at the #2 position replacing its H. The freed #2 hydrogen is picked
up by the free bond of #6 O.
Bond changes: break O-H bond and half a C=O bond (other half of C=O bond changes to C-O bond), make a C-O bond and an O-H bond. The O-H bonds wash, so the energy difference between the linear and ring forms is [2 x 93.5 (C=0 per electron) - 2 x 78 (C-O) = 31 kcal/mole]. The carbon sinks closer to the oxgyen in a double bond, so the linear form, which in a sense more CO2 like (in the confusing terminology has higher bond energy), but it should be the more stable form (see below).
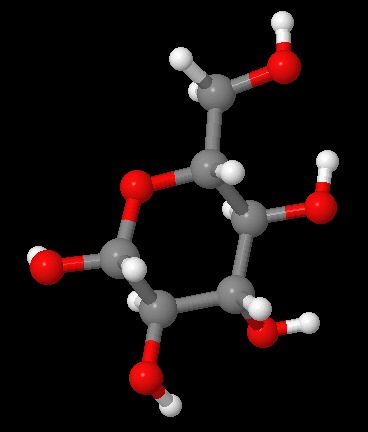
Ring form of D-glucose
(source -- http://www.worldofmolecules.com/3D/glucose_3d.htm)
Models at this site can be rotated
As shown below, the difference in energy between the two forms is small (1.4%), so apparently it easily converts back and forth. Linear and hex loops coexist, in fact 99% of glucose in solution is the loop back form. So it's probably not too surprising when I looks at the details of the glycogen chains, I see it is chains of looped glucose.
Linear chain
Ring form (5C + O)
----------------------------------------
-------------------------------------
5 C-C
5 x 80 = 400
5 C-C
5 x 80 = 400
7 C-H
7 x 98 = 686
7 C-H
7 x 98 = 686
5 C-O
5 x 78 = 390
7 C-O
7 x 78 = 390 + 156
1 C=O 1 x 187
= 187
0 C=O
0 x 187 = 0
- 187
5 O-H
5 x 110 = 550
5 O-H
5 x 110 = 550
----------------------
-------------------
total 2,213 kcal/mol
total 2,213 - 31
What?Linear glucose chain
Wait, I don't understand this. The energy number indicate the linear form should be the more stable! It predicts enegy will come out when a ring form converts to a linear form (as a CO2 like C=O bond is made). Wikipedia (glucose) says the open-chain form is thermodynamically unstable. Are there are other energies stored in rotation, etc? These two forms of glucose are called 'tautomers' and the interconvertability 'tautomerization'.
Folded back (ring) glucose in glycogen
An O is shared
between two glucose when they are chained in glycogen. Each glucose has
lost one O and one H, so glucose in glycogen changes from C6H12O6 => C6H11O5.
The texts don't say so, but if the metabolism processes need full glucose
to work (they may not), then water must be supplying the missing H and
O as glycogen is disassembled into glucose.
----------------------------------------------------------
Cellular respiration
Cellular respiration
is the name for long sequence of reactions used by cells to 'burn' glucose
to get energy in the form of ATP. Wikipedia has a figure giving a summary
of the whole process. While it's obviously quite complicated, it can be
separated into a front end process and two back end processes.
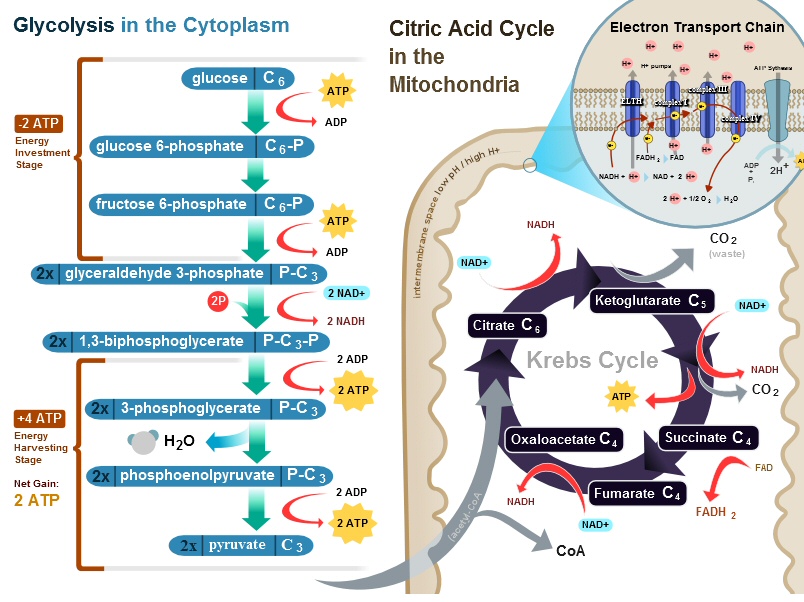
Cellular respiration in eukaryotic cells
(This figure was fixed after I emailed the author
about an error I found in the lower left corner,
pyruvate was shown with a P, see Appendix, May 2011)
Back end
Inside the mitochondria
run two back end processes. The first is the krebs cycle which reduces
NAD+ to make NADH. NADH then powers the 2nd back end process: an electron
transport chain which pumps protons into the inner membrane space. The
proton gradient is then used by the rotating machine ATP synthase to make
ATP.
Irony (glucose => fructose)
Look at the
figure below, which shows the entry of glucose into the front end glycolysis
process. Glucose enters (in ring form) and has a phosphate (P) added, next
glucose (with P) is converted to the five ring form of fructose (with P)!
Sure fructose is only an intermediate on the way to pyruvate, but still
glucose the king (?) of sugars is (apparently) always converted
to the simple sugar fructose before being burned! The implication (to me)
is that fructose could probably work as a cell energy source, but my guess
is that either there is a problem transporting it in the blood, or regulation
and control of a dual sugar system in the body is just too complicated.
Glucose vs pryuvate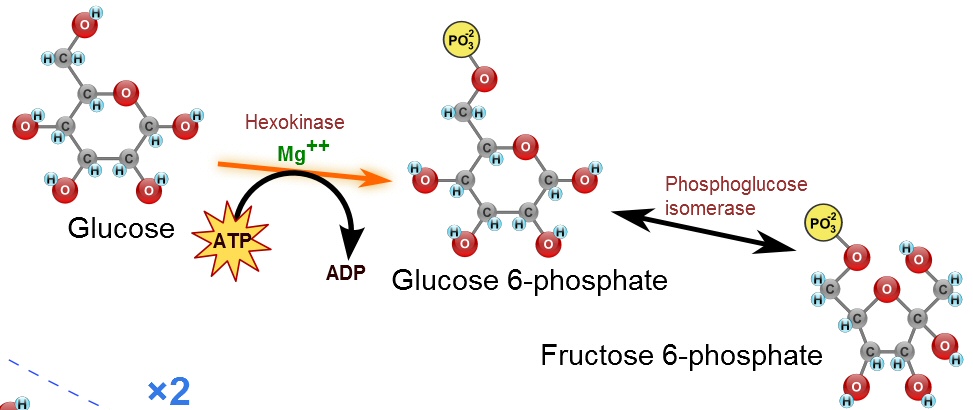
ringed glucose converted to ringed fructose (with P) near beginning of glycolyis process
(double arrow indicates a reversible process)
source -- Wikipedia 'Glycolysis'
 .
. 
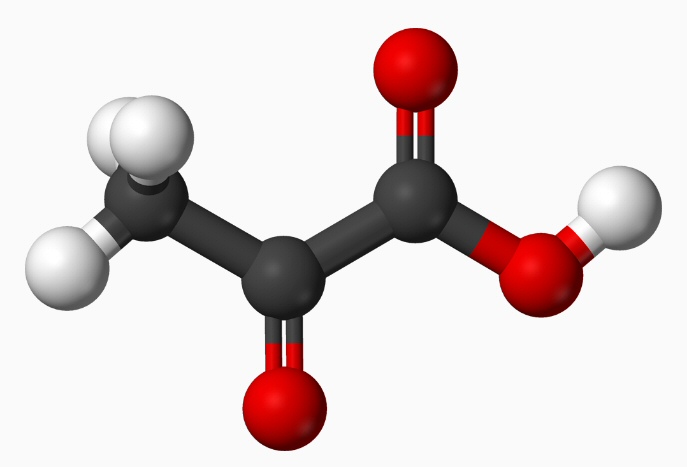
Pyruvic acid -- three carbon chain
2 pyruvate (in kcal/mol)
The bond energy
in two pyruvate is 181 kcal/mol lower than glucose (calculated above)
2 x 2 C-C
4 x 80 = 320
2 x 3 C-H
6 x 98 = 588
2 x 1 C-O
2 x 78 = 156
2 x 2 C=O
4 x 187 = 748
2 x 1 O-H
2 x 110 = 220
----------------------
total 2,032 kcal/mol (bond energy)
-------------------------------------------------------------------------------------------------------------------------------------------------
Oxidation (in air)
All the references
I see for calculating energy of oxidation are just mechanical, plug in
the bond energy numbers of the two sides of the equation and subtract the
difference. But I wanted a more intuitive understanding, a way to see how
the oxidation energy varies with food's (or fuel's) chemical structure,
a way to estimate the energy without a full calculation. After thinking
about this a while, I came up with a few ideas (have not seen these ideas
anywhere else).
1) Good way to look at bond energy numbers in summary
equation
A good
way (I think) to look at oxidation of food or fuel (molecules of C, O,
H) in air is to first find the gross energy yield. This is the right
side bond energy (in CO2 and H2O) minus the bond energy in the food or
fuel molecule (at this point ignoring the O2), then subtract off the energy
cost,
which is the energy required to break the covalent O2 double bond. The
result in the net energy release (as heat).
2) Find fraction of food energy released in oxidation
How good a
fuel
a food molecule is can be assessed (I suspect) by ratioing the net
released bond energy to the bond energy in the food molecule. This tells
you what fraction of the food (bond) energy is released when the food is
burned.
3) Estimate the energy release from the amount of external
oxygen used!
I kept looking
at the energy numbers of C,H,O bonds that are broken and remade in CO2
and H2O to see if I could really figure out how oxidation works.
Isn't there a way to estimate the energy output of a (simple) food or fuel
molecule without having to mechanically do the bond energy calculations?
EE engineers can often tell a lot about a circuit just by looking at its
schematic, can't chemists do this with oxidation reactions?
I kept thinking the energy release should be estimable from energy difference of the fuel/food C,H,O bonds vs the output CO2 and H2O bonds (C=O and H-O). It seemed obvious (initially) that the energy output must be coming from the C and H bond energies going up as their electrons sink deeper into the energy well of O.
From (per electron)
To (per electron)
C-C 80
C=O 93.5
(CO2)
C=C 72.5
H-O 110
(H2O)
C-O 78
-----------------------------------
C-H 98
H-O 110
The hydrogen bond [C=H, 98] increases in energy a little (12%) when hydrogen rebonds to oxygen in water [H-O, 110]. Carbon bonds [C-C, 80; C=C, 72.5; C-O, 78] increase in energy moderately (21% average) when carbon rebonds to oxygen in CO2 [C=O, 93.5]. But carbon bonded to hydrogen [C-H, 98] loses energy (-5%) when it rebonds to oxygen [C=O, 93.5] in CO2. Hydrogen in food bonded to oxygen [H-O] contributes nothing to energy output because the hydrogen bond in water is the same. (Same would be true for any C=O bonds that might exist in food or fuel.)
Well the bond energy numbers look higher, but not by much. Then it hit me .......

External O2 makes more bonds (sort of)!
The big energy
gain in oxidation is not the reconfiguring of the electron bonds
of the food/fuel to the bonds of CO2 and H2O, but the fact that CO2 and
H2O will have substantially more bonds than the food/fuel molecule.
When C and H split apart, each of these atoms are now free to join
up with an external O (pulled from the O2 in air)! More bonds are
made. Splitting of a single C-H bond in food/fuel ends up creating
an O-H bond in water and half a C=O bond in CO2! For example, a
glucose molecule (C6H12O6) has 24 (electron) bonds, but when oxidized the
six
CO2 and H2O it forms have 36 bonds, 50% more bonds (and on balance slightly
higher energy bond too)!
Another viewpointSplitting O2 is easy
Perhaps a better way to think about it is this. The amount of CO2 and H2O, and thus the number of bonds on the output and input sides of the equation, is determined by the amount of carbon and hydrogen in food. What oxygen in food does is displace low energy bonds in oxygen gas (O=O, 58 cal/mole per electron) with high energy carbon and hydrogen bonds from the food (C-O, 78; C=O, 93.5; H-O, 110). Two of these food oxygen bonds (C=O, H-O) are the same as in output CO2 and H2O, so only the food oxygen C-O bond, which gets upgraded to C=O in CO2, contributes any energy (78 => 93.5 cal/mole per electron). (Are there any O-N, or O=N bonds in proteins?)
How much O2 predicts energy output!
The previous
paragraph hints it might be possible to estimate energy output of
an oxidation reaction by just looking at how much oxygen is coming in from
the air! The reason is that the O2 covalent bond cleaves relatively easily
and when the oxygen recombine with the H and C the energy per electron
is almost doubled (1/0.57 = 175%). In a summary equation not only must
the atoms on both sides of the equation balance, but the bonds (count per
electron) must balance too. For example in glucose oxidation there are
36 electron bonds on each side of the equation: on the left, 24 in single
glucose molecule and 12 in 6 O2, and on the right, 24 in six CO2 and 12
in six H2O. In methane oxidation there are 8 electron bonds on each side:
4 in CH4 and 4 in 2 O2, and 4 in CO2 and 4 in two H2O.
Test --- Find the energy release per O2 (electron)
bond
If my guess
is right, then dividing the net energy release of the oxidation reaction
by the number of electrons in external O2 should give us numbers in the
same ballpark. And this estimate would predict the energy release is something
like a little less than the total energy in all the external O=O bonds
of the external oxygen, since the external oxygen bond energies are upgraded
by about 175% (in CO2 and H2O) plus a little extra energy from bond upgrades
within the food/fuel. Here's the result
Methane
190 kcal/mole release, 2 O2 => 47.5 kcal/mole (= 190/4)
per O2 electron
Glucose
655 kcal/mole release, 6 O2 => 54.6 kcal/mole (= 655/12) per
O2 electron
two pyruvate
512 kcal/mole release, 5 O2 => 51.2 kcal/mole (= 512/10) per
O2 electron
The ratio [energy out/(# of external oxygen electrons)] averaged is 51 kcal/mole +/- 7 % for the three cases I examined. Nicely clustered. And 51 kcal/mole out is 88% of the 58 kcal/mole bond energy of each electron in O=O.
It works!
I would say this
'O2 estimate' of energy release works pretty damn well! Oxidation of two
half glucose (two pyruvate) pulls in 5 O2 (from air) and releases (as heat)
512 cal/mole. The release energy is just a little less than the 580 kcal/mole
(= 10 x 58 kcal/mole) contained within the O=O double bonds of the five
molecules of O2 pulled in from the air.
Effect of oxygen in food/fuel
A 64 dollar
question is how does the presence of oxygen in a food/fuel affect its oxidation
energy output? Clearly this is important in food as sugars (like glucose,
C6H12O6) have a lot of oxygen. In fact since oxygen is heavy (O atomic
weight 16 vs CH2 of 14) more than half the weight of sugar is oxygen!
From the previous discussion the reconfiguring of oxygen bonds in food/fuel to those in CO2 and H2O do produce a little energy. The upgrade of a single C-O bond to a double C=O has about a 20% energy increase (78 => 93.5 kcal/mole), but oxygen bonded to hydrogen (O-H) in food/fuel yields no energy output, because all the hydrogen in the food/fuel will end up in water with the same (O=H) bond.
No, the big energy impact of oxygen in food/fuel is a reduction in the amount of external oxygen that must be pulled from the air, and this is the source of most of the energy release in oxidation. For example take glucose (C6H12O6). Its six carbon go to six CO2 and to absorb its twelve hydrogen six H2O are needed. (6 CO2 + 6 H2O) require 18 oxygen, but only 12 need be pulled in from the air, the other 6 coming from the sugar.
Thus using the 'external O estimating rule' my guess is that oxidation of sugar (C6H12O6) will yield about 2/3rd of the energy (per molecule) of oxidation of a pure hydrocarbon (no oxygen). And when weight is considered this is 2/3rd of the energy for x2 weight. So my conclusion is that any food or fuel with a lot of oxygen, like glucose, when oxidized is going to output, per unit weight, something like 1/3rd the energy of a pure hydrocarbon (like methane, CH4).
Check:
methane:
16 gm/mole, 190 cal/mole
190/16 => 11.9 cal/gram
glucose
180 gm/mole, 655 cal/mole
655/180 => 3.64 cal/gram
Wow:
The ratio
is (3.64/11.9) = .306, pretty damn close to the 1/3rd my 'external O2'
rule predicted. It works!
Three oxidation examples
Glucose, (two)
pyruvate (sort of half glucose), and methane
a) Oxidation of methane
CH4 + 2O2 =>
CO2 + 2H2O
4 x 98 2 x 116 =>
2 x187 2 x 2 x 110
392 + 232
=> 374 +
440
624 => 814
(190 cal/mol energy released)
Gross
Yield
814 - 392 = 422 cal/mole
Net
422 - 232 = 190
net/food
[190/392] = 48% (methane is an excellent fuel)
b) Oxidation of glucose
C6H12O6 + 6O2
=> 6CO2 +
6H2O
2,213 6 x 116
=> 6 x 2 x187 6 x 2 x 110
2,213 +
696 => 2,244
+ 1,320
2,909 => 3,564
(655 cal/mol energy released)
Gross
Yield 3,564
- 2,213 = 1,351 cal/mole
Net
1,351 - 696 = 655
net/food
[655/2,213] = 30%
c) Oxidation of two pyruvate
Two puruvate
(eq C6H8O6) have four less H than glucose (C6H12O6), thus it yields two
less water (where the big energy gain is) although the cost is a little
less (one less O2 must be broken).
2 C3H4O3 + 5O2
=> 6CO2 +
4H2O
2,032 5 x 116
=> 6 x 2 x187 4 x 2 x 110
2,032
580 =>
2,244
880
2,612 => 3,124
(512 cal/mol energy released)
Gross Yield
3,124 - 2,032 = 1,092 cal/mol
Net
1,092 - 580 = 512
net/food
[512/2,032] = 25%
I was right
oxidation of two pryuvate yields less energy than oxidation of one glucose
molecule {78% = (512/655) of the energy of burning one glucose}. The 22%
loss can be explained as sum of two factors: one, there is 9% less (bond)
energy in the two pryuvate (2,032/2,213), and two the pryuvate oxidizes
less efficiently (due I think to it containing less hydrogen, which means
less water) releasing 25% of its bond energy vs 30% for glucose, a ratio
of about 0.85 (= 25%/30% approx).
-------------------------------------------------------------------------------------------------------------------------------------------
What is food, really, at the chemical level? I am not talking about the small quantities of minerals, like sodium, calcium, potassium, iron, chlorine, etc, needed to build the body and run the biochemical machinery. Nor am I talking about tiny quantities of chemicals, like vitamins, we need but either cannot produce (or produce enough of). I am talking about the major benefit food provides, which is energy, the energy required to maintain our body temperature, the energy supply so we can dissipate continuously 75 to 100 watts of energy. Mostly what food does is provide energy (in the form of calories)!
My first reaction
was to say food is just various combinations of three atoms: (C,H,O) carbon,
hydrogen, and oxygen. Certainly this is what some food is, like sugars,
(C6H12O6). And sugar (glucose) is what photosynthesis outputs, so this
(from an energy output point of view) is what virtually all plants
(and photosynthetic bacteria too) live on.
-----------------------
Summary
* Food in
terms of energy (calories) is only C,H and O. In fact nearly all
the energy comes from the carbon and hydrogen of food. Oxygen bonds in
food contribute little energy. The C-O bond in food when upgraded to C=O
in CO2 contributes some energy (78 => 93.5 cal/mole per electron). The
C=O and H-O bonds in food contributes no energy as they are the
same bonds in the outputs CO2 and H2O.
* On balance oxygen in food reduces energy. One, oxygen is heavy (atomic weight 16, vs 12 for carbon and 1 for hydrogen) so energy per unit weight of food is reduced. Two, oxygen in food reduces the carbon and hydrogen energy output because less external oxygen is required during oxidation. The low energy bonds in oxygen gas (O=O, 58 cal/mole per electron) are displaced by high energy oxygen bonds with carbon and hydrogen (C-O, 78; C=O, 93.5; H-O, 110). Only the C-O bonds contribute any energy, because C=O and H-O bonds are the same in CO2 and H2O.
* Food is mostly C,H and O configured as sugar, fat and protein. Sugar and fat are made up only of carbon, hydrogen and oxygen. Sugar (C6H12O6) is roughly 50% oxygen by weight. There is only a little oxygen in fat. The formula for fatty acid or triglyceride is C55H98O6, so by weight it is roughly 10% oxygen. Sugar due to its high oxygen content has less than half the calories per gram compared to fat (4 vs 9 cal/gram).
* At least one N is included in all amino acids, which strung together make up all proteins, so protein food (from plants or animals) includes nitrogen (plus a smudge of sulfur). Protein is about 85% C, H, and O plus 15% N. Two amino acids have one atom of sulfur, so there is a tiny bit of sulfur in protein too. (And a tiny amount of selenium too, because one rare amino acid has one atom of selenium.) Nitrogen in food is used to replace lost nitrogen (protein) from the body (skin, hair, sloughed off GI cells, etc). It is not used for energy.
* Vitamins are small molecules of C,H,O (sometimes including an S or other minerals) that function as enzymes. A few protein enzymes in some reactions need a co-enzyme (a small molecule to hook up to to function), and this is what vitamins are. A classic example is vitamin A, which provides (a precursor to) retinol which embeds in a protein in the retina to sense light.
* The
energy (calories) in food comes almost entirely from its carbon and hydrogen
content via oxidation, yielding CO2 and H2O. While the biological processes
that do the oxidation are complex, the food energy yield is the same as
though the food were burned.
-----------------------
Nitrogen
But I forgot
about nitrogen, the other atmospheric gas. There is no simple formula for
protein, a major component of the diet for those who eat meat, as there
are thousands of proteins. Proteins are long strings of the twenty or so
amino acids linked with a backbone made of carbon and nitrogen. The basic
formula for an amino acid shows an N, so it looks like all amino acids
probably have at least one nitrogen atom. A google serch finds the
nitrogen content of protein (food) is about 15% to 16%. So what (if anything)
does the nitrogen contribute to the oxidation of food?
What's in amino acids anyway?While plants 'feed' on glucose, they need nitrogen, in the form of nitrate or ammonium pulled up by roots from soil, to combine with the glucose to make proteins. Don't know all the purposes of proteins in plants, but proteins are enzymes required to make nearly all bichemical reactions go. Proteins must make up a substantial fraction of the bulbs, seeds or leaves of the plants we eat for food, because there is a lot of plant protein in the plant products we eat. Certainly animals are essentially made of protein. Nitrogen is also a component in the nucleic acid of each cell, but I suspect from an oxidation point of view this is a tiny component.
The best reference I found for amino acids is a chart in Wikipedia 'Amino Acid' showing that eukaryotes (i.e. non-bacteria) proteins are built from 21 amino acids. I paged through all 21 amino acids. All but three are composed of just four atoms: carbon, hydrogen, oxygen and nitrogen. Most have just one nitrogen, a few have 2, and a couple 3 or 4. Since amino acids are small molecules, one N can be 10%-15% of the amino acid by weight. 2 of the 21 amino acids have a single sulfur atom (in addition to C,H,O,N), and 1 of 21 (probably pretty rare) has a single atom of selenium!
However, I doubt that we are oxidizing nitrogen to NO2 in the body (or phosphorus either)! My guess is that much of the nitrogen we eat is stiped away by the liver and/or kidney and extreted in urine with a needed fraction (in form of NH3, ammonia) kept for builiding proteins and DNA.
** -- A healthy body is characterized by a nitrogen equilibrium (steady state equilibrium) where intake and loss of nitrogen are equal. The interpretation would be that the body uses only as much protein nitrogen from the diet as necessary to replace digestive enzymes, gastrointestinal cells (GI) lost in the feces, or any degenerated tissue components, such as skin cells or erythrocytes that wear out during normal use.
-- Animal proteins are a better mix for our diet than plant proteins based on their amino acid composition, but protein in the form of hair and skin keratin is non-digestible and useless as such.
-- Nucleic acids, on the other hand, are not needed as dietary supplement. Excess nucleic acid in the diet is degraded and secreted and most nucleic acid synthesis in cells is provided by protein degradation.
-- Urine contains large quantities of nitrogen (mostly as urea), as well as significant quantities of dissolved phosphates and potassium, the main macronutrients required by plants.
-- Amino acids from ingested food which are not used for the synthesis of proteins and other biological substances are oxidized by the body, yielding urea and carbon dioxide, as an alternative source of energy. (Wikipedia, Urea, Ref: "Amino acid metabolism")
Nitrogen balance
The first reference
above is the key one. It says we take in net nitrogen when growing,
but a steady state condition means on average all the nitrogen being taken
in in food is lost by a variety of mechanism. The nitrogen taken in is
used to replace nitrogen lost in feces, skin cells sloughing off, hair
growing, and also (from Wikipedia) excretion in urine of numerous by-products
of cellular metabolism pulled from the blood, many rich in nitrogen. The
excess nitrogen in the diet not aborbed for use building proteins, etc,
is probably quickly excreted as urea (2N4HCO) in urine.
Plant nutrients
One gardening
reference categorizes plant nutrients this way:
Carbon, hydrogen and oxygen
- Available from air and water
Nitrogen (N), phosphorus (P), potassium (K)
- Macronutrients, in packaged fertilizers
Sulfur, calcium, and magnesium
- Secondary nutrients (available in soil)
Boron, cobalt, copper, iron, manganese,
molybdenum and zinc
- Micronutrients
-- Every amino acid contains nitrogen
-- Every molecule making up every cell's membrane contains phosphorous
(membranes and ATP)
-- Potassium makes up 1 percent to 2 percent of the weight of any plant
(essential cell ion)
Composition of human body (by weight)
For a 70kg
body 1 gram is 0.0014%, so zinc at 0.0032% is 2 grams in the body.
Oxygen (65%), Carbon (18%), Hydrogen (10%), Nitrogen (3%), Calcium (1.5%),
Phosphorus (1%)
Potassium (0.25%), Sulfur (0.25%), Sodium (0.15%), Chlorine (0.15%)
Magnesium (0.05%)
Iron (0.006%), Fluorine (0.0037%), Zinc (0.0032%)
-----------------------------------------------
Elements below
are at less than 1 gram. Copper at 0.0001% is 70 mg.
Copper (0.0001%)
Iodine (0.000016%), Selenium (0.000019%), Manganese (0.000017%), Molybdenum
(0.000013%)
Chromium (0.0000024%), Cobalt (0.0000021%)
http://en.wikipedia.org/wiki/Composition_of_the_human_body#Atoms_in_the_human_body
Fats
Fats are high
calorie/gram food (9 cal/gm vs 4 cal/gm for sugar and protein), because
most of the oxygen is missing, they are close to being pure hydrogen carbons.
For example, a triglyceride is C55H98O6 with only about 1/10th oxygen relative
to carbon as in sugar. What 'saturated' means in terms of fat (saturated
and unsaturated fat) is that the carbon is saturated with hydrogen.
In a saturated fat the inside carbons couple with C-C bonds so each
is decorated with two hydrogen, whereas in unsaturated fats carbons are
coupled with C=C bonds. Cholesterol is a waxy steroid of fat (C27H46O).
Vitamins
All my life
I have never known what vitamins do, but a little tolling around Wikipedia
and I think I understand. There are 13 of them and (apparently) they only
do one thing, they are enzymes. Virtually all biological reactions require
enzymes to go. Nearly all enzymes are proteins, but the protein enzyme
for some reactions require an attached small molecule to work. This is
what vitamins are, they are the attached small molecule, technically they
are a co-enzyme. Like amino acids some vitamins can be manufactured in
the body (vitamin D by sunlight on the skin, how is that for exotic!) and
some can't, these are the 'essential vitamins' and must be part of the
diet. Some vitamins are organic and some inorganic, some are water soluable
and some are fat soluable. Fat soluable vitamins in the diet are found
only in fat, one reason fat in the diet it essential.
Wikipedia 'Vitamin' is full of weird facts about vitamins. Vitamins names have changed, various substances have been classified as vitamins and then unclassified as vitamins. Salicylic acid (Vitamin S, aspirin) has been proposed as a vitamin. The recommended daily quantity of most vitamins in the diet is ug to mg. There are anti-vitamins in some foods, part of a class of anti-niutrients, that interfere with action of some vitamins. One reason food is cooked is to reduce its anti-niutrients. Quite a few studies are referenced on the dangers of vitamin supplements. The liver stores enough of three vitamins (A, D, B12) for months to years. For a vitamin listed as not stored in the human body (B3) the body can a two weeks supply. It takes a month to six months before lack of vitamin C causes symptoms of scurvy.
What are vitamins
made of? Most are carbon, hydrogen, oxygen with some nitrogen. Vitamin
A (retinol, needed by retina of the eye) is just carbon, hydrogen and oxygen
(C20H30O). Vitamin B1 is thiamine and contains one sulfur (prefix 'thio'
means sulfur containing). Vitamin B6 has on phosphorus. Vitamin C is C6H8O6,
chemically it is just sugar (C6H12O6) minus 4H!
------------------------------------------
(from photosynthesis essay)
Ref: 1 ev = 23.1 kcal/mol = 96.5 kj/mol
Oxidation of glucose to make ATP------------------------------------
** -- If glucose is simply burned in air, all of this energy is released as heat.C6H12O6 + 6 O2 => 6 CO2 + 6 H2O + 686 kcal/mol (29.7 ev)
In a cell, however, oxidation of glucose is coupled to the synthesis of ATP from ADP in the following reaction:
C6H12O6 + 6 O2 + 36 ADP + 36 Pi => 36 ATP + 6 CO2 + 6 H2O
Efficiency of this reaction
Oxidizing one molecule of glucose in air yields 29.7 ev of heat. When glucose is oxidized to make ATP (from ADP + Pi) we get 36 ATP (plus 6 O2 and 6 H2). The available energy in ATP (when transformed to ADP + Pi) is 7.3 kcal/mol (0.316 ev). So efficency of turning glucose energy into ATP energy is[ 36 x 0.316 ev (ATP)]/29.7 ev (glucose) = 38.3%
Ref not too clear but above is apparently for equal concentrations of ATP and ADP. ATP concentration in cells can by x5 or x10 ADP, then 7.3 kcal/mol can rise to 12 kcal/mol, which increases efficiency from 38% to 50% or so (number not right).
So I am responsible for correcting an error in the biochemistry this figure in Wikipedia (May 2011).
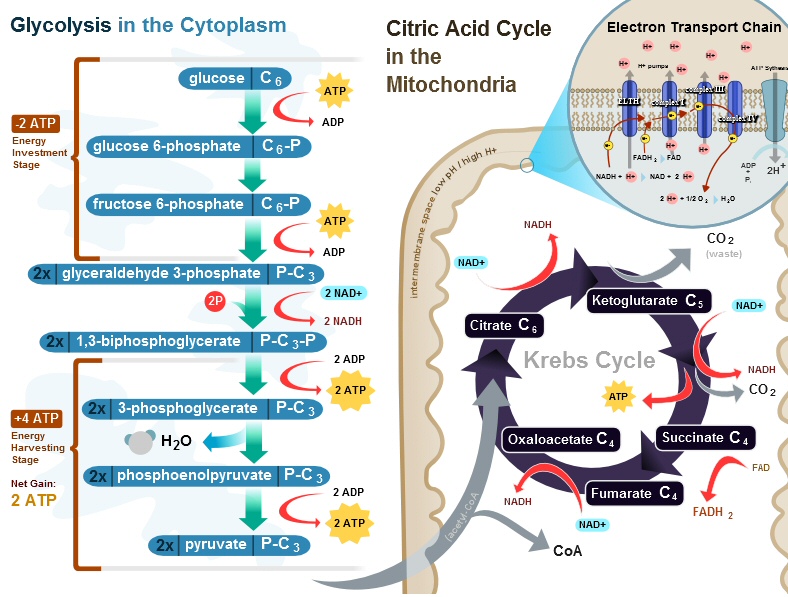
(I am pretty sure there in an error in the lower left
corner, which shows the shorthand for pyruvate as P-C3
Pyruvate has no P (phospate group) and from above
the P in phosphoenolpyruvate leaves with ATP)
I provided feedback on this to the author: Regis Frey
source --- Wikipedia 'Cellular respiration'